Dalia Savy
A
Anika P
AP Chemistry 🧪
269 resourcesSee Units
Structure
As mentioned earlier, ionic interactions can produce brittle, hard solids that have high melting points. This is due to the ions being held in a 3D array, known as a crystal lattice.
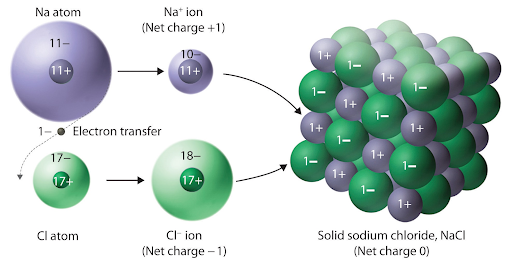
The reason for this is that ions are attracted to their opposites, and so negative ions surround positive ones and vice versa.
It is important to note that particle diagrams for ionic substances look differently than molecular substances. Covalent substances are usually represented by a molecule while ionic substances are represented by a network of positive😊 and negative😞 ions.
Explaining the Lattice Structure
The lattice structure can be explained by the strong electrostatic forces:
E = kQ1Q2/d
- Q1, Q2 = charges on the particles
- d = distance between centers
- k = constant 8.99 x 109 J-m/C2
- energy increases as charge increases or when distance between atoms decreases
💡Attractive forces between cations and anions are maximized in the lattice structure while repulsive forces are minimized.
Properties of Ionic Substances
In a lattice, electrons are stuck in place, or in other words, they are localized. This makes ionic solids very poor conductors of heat and electricity since there are no electrons moving around.
When ionic solids melt into liquids though, they do conduct heat and electricity. This is because the ions are free to move in the liquid phase. This applies to the aqueous phase as well.
Lattice Energy
Lattice Energy is the energy released when ions bond to form an ionic solid. Remember Coulomb's Law? It actually relates to lattice energy too; it's kind of everywhere.
Review
To recall, Coulomb's Law states that the smaller the distance and the higher the charge, the stronger the attraction between two ions💪.
Let's ease into lattice energy...do you also remember how to find out which ionic compound would have a higher melting point? Let's review that, it'll help with lattice energy I promise!
- Out of NaF and NaCl, which one has the higher melting point?
- First, we would look at the charges of the ions. Here, they are both +1/-1, so charges can't have an effect on the differences in melting point. Then, we would look at which ions are smaller. Remembering the periodic trends, F- is much smaller than Cl-. Since it is smaller, it can be closer to the Na+ ion and increase the strength of attraction. Therefore, it takes more energy to break the bond, increasing the melting point.
If you still don't remember that process, you can review here!
Putting Everything Together
Lattice energy depends on the same two concepts that you used in that question: charge and distance. Coulomb's Law directly relates to melting point and lattice energy so just remember:
The smaller the size and the higher the charge, the higher the lattice energy🤔. Therefore, the higher the melting point of an ionic solid, the higher the lattice energy.
Easy rule, right? Let's try a few out:
Which of the following compounds have a higher lattice energy?
- NaF or NaCl
- Since we already thought this question through, it's easy to tell that NaF has the higher lattice energy.
- MgO or NaF
- Charge first: Mg and O have a +2/-2 charge, while Na and F have a +1/-1 charge. You don't even have to check distance, MgO must have the higher lattice energy.
- NaF or KCl
- Since these ions are in the same groups, they have the same charge. We must remember the periodic trends and that the farther down in a group you go, the larger the ions get. Therefore, K+ and Cl- are larger than Na+ and F-. With the smaller size, NaF has the higher lattice energy.
- LiCl or NaCl
- Last one! Since Li+ is smaller, LiCl must have the higher lattice energy.
Check your Understanding
The following question is from the Advanced Placement YT Channel. All credit to them.
- Answer the following questions related to Mg and Sr.
- Write the complete ground state configuration for the ions Mg+2 and Sr+2.
- Do you predict that the ionic radius of Sr+2 is larger or smaller in size than the ionic radius of Mg+2? Justify your answer in terms of atomic structure and the electron configuration of each ion.
- The lattice energy of MgCl2(s) is equal to 2300 kJ/mol. Do you predict that the lattice energy of SrCl2(s) should be less than or greater than 2300 kJ/mol? Justify your answer in terms of Coulomb's law.
Sample Responses
(a) Looking at the periodic table and remembering electron configuration, you should get:
You cannot leave the electron configurations of Mg and Sr as your final answer! Make sure you always answer what they are asking.
I originally wrote down the electron configurations of Mg and Sr and then took off two valence electrons to get the final electron configurations of Mg+2 and Sr+2.
(b) This question goes back to periodic trends. Which ion has more electrons and electron shells? Sr2+ does, so it has the larger ionic radius.
Sample Response: Sr+2 has a larger ionic radius than Mg+2 because it has more occupied electron shells. The valence electrons in Sr2+ are in the 4th energy level whereas the valence electrons in Mg2+ are in the 2nd energy level. Electrons in the 4th energy level are generally farther away from the nucleus, making the ion larger.
(c) Charges are the same, so size must be accountable for the difference in lattice energy.
Sample Response: Coulomb's law states that the higher the charges of the ions and the smaller the distance between the ions, the stronger the attraction and the higher the lattice energy. Although the charges of Mg and Sr are the same, Sr is a much larger ion due to its greater amount of occupied energy shells. Since it is larger, the distance between Sr+2 and the chlorine ions is greater than the distance between Mg+2 and the chlorine ions. Therefore, the lattice energy of SrCl2 (s) must be less than 2300 kJ/mol.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.