Dalia Savy
A
Anika P
AP Chemistry 🧪
269 resourcesSee Units
Resonance
If a structure can have multiple ways of drawing a Lewis structure, the structure is known to have resonance. A good way of thinking of resonance is like mixing paint🎨.
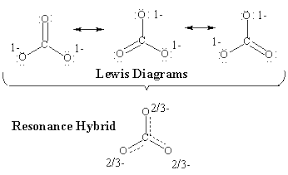
When you draw out the two or three resonance structures of a molecule, those two/three structures make up the entire molecule. The actual structure is represented by an average of these 2-3 structures. This can lead to bond orders that are fractions, such as bond orders of 1/3 or 2/3.
How do you know when something has resonance?
Let's try drawing the lewis dot structure (LDS) of NO3-.
Recall from the last guide, here are some steps:
- Count the number of valence electrons. Nitrogen has 5, oxygen has 6. 5+6+6+6 = 23. But...NO3- is a polyatomic ion and there is a charged attached to the molecular as a whole. The -1 charge indicates that there is one more electron, so there must be a total of 24 valence electrons represented in the LDS.
- Draw the central atom, which in this case, is Nitrogen.
- Draw the 3 oxygen atoms surrounding Nitrogen and single bonds connecting them. Draw the full octets:
- Count the current number of valence electrons. There are 26. Somehow we have to get rid of 2 of them🤔. Remember, when there are too many electrons, we can get rid of the single bond and put a double bond. In this case, if we change them all to double bonds, we won't have enough electrons! This is where resonance comes in: you only change one to a double bond.
- 💡*Remember, for polyatomic ions, you must put brackets and the charge*
One representation of NO3-
Now we count: we have 24! This is one way to draw the lewis dot structure of NO3-.
How do we know which bond to make a double bond?
Simple answer: any of them! There are three ways to draw this structure:
Any of these would be acceptable. On the AP Exam itself, it is good to draw all three side by side with arrows between them ↔️.
In reality...🤔
In reality, the structure doesn't have 2 single bonds and 1 double bond. It has a 4/3 bond order. There is just no way to represent 4/3s of a bond.
Bond Order?
How did I know it was a 4/3s bond? The easiest way to know the bond order is ( # of lines / # of spaces ). There are 4 lines or bonds and 3 spaces for bonds. A 4/3s bond is between a single bond and a double bond. Therefore, it is stronger than a single bond, but weaker than a double bond.
💡As mentioned before, on the AP Exam, draw all possible structures and just write the word resonance to clarify what you know to the AP Grader.
⭐Try drawing the LDS of nitrite (another polyatomic ion)
Formal Charge
Formal charge is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms. It reflects the electron count associated with the atom compared to the isolated neutral atom. It is used to predict the correct placement of electrons.
Here is an overall diagram, but let's get into the nitty gritty.
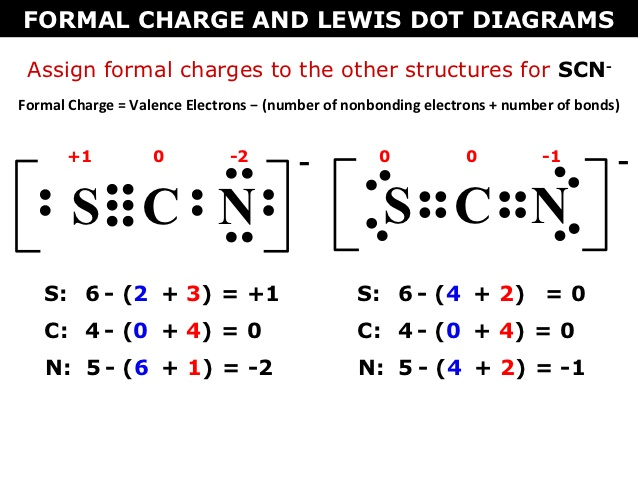
How do you calculate formal charge?
The easiest way to calculate formal charge is to do (# of valence electrons - # of dots - # of dashes). This might sound weird but it's just the easiest way to remember it!
Look at the example above at the SCN- ion to the left and the way they calculated formal charge.
S: They took the number of valence electrons (6) and subtracted it by the total number of lone electrons (2) and single bonds (3 [there is really a triple bond]).
When do I check formal charge?
It is good to always check formal charge, especially since it is easy. If anything, just check it if an element past element 14 is involved (because that is when you can break the octet rule).
Example
Let's draw the LDS of phosphate (PO4-3):
- There should be 5+6+6+6+6+3 valence electrons, so 32 total.
- Draw Phosphorus in the center and 4 oxygen atoms surrounding it with single bonds and full octets:
- Looking at this lewis dot structure, we count 32 valence electrons and think it is perfect! However, Phosphorus is element 15 so we should be looking at formal charge to make sure the placement of electrons is correct.
- Calculating the formal charge:
- P: 5 valence electrons - 0 dots - 4 bonds = +1 formal charge
- O: 6 valence electrons - 6 dots - 1 bond = -1 formal charge
- When we are looking at the formal charges, we want the central atom to always have a formal charge of 0. A formal charge of 0 means the electrons are localized, or not moving.
- One way we can lower the formal charge of Phosphorus to 0 is by adding a double bond:
- Now let's check formal charge once more:
- P: 5 valence electrons - 5 bonds = 0 (Perfect🥳)
- Top 3 Oxygen atoms: 6 valence electrons - 6 dots - 1 bond = -1
- Botton oxygen atom: 6 valence electrons - 4 dots - 2 bonds = 0
Now, instead of having no atoms with a formal charge of 0, we have two atoms with a formal charge of 0. The electrons are placed correctly, resulting in a stable bond🎉.
The ion also now as resonance, so you should draw all 4 ways of representing it and know the bonds are 5/4 order.
💡Quick Tip: An easy way to check if you calculated formal charge correctly is if the sum of the formal charges = the charge of the ion.
Since there were 3 oxygen atoms with a -1 charge, the sum is -3, which is equal to the -3 charge of the polyatomic ion.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.