Dalia Savy
AP Chemistry 🧪
269 resourcesSee Units
If you recall, precipitation reactions are one of the main three you should know for this unit. Let's review it once more!
Precipitation Reactions Explained
When ions aqueous solutions react, they may produce an insoluble (undissolvable) or barely soluble solid ionic compound. This solid product is called a precipitate.
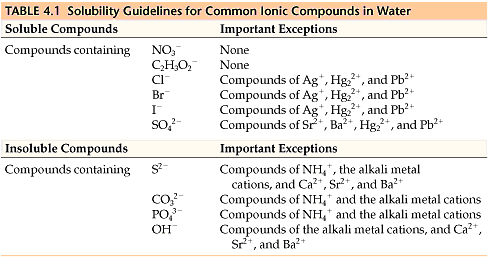
All sodium, potassium, and nitrate salts🧂 are soluble in water, so they aren’t precipitates. You don’t need to know any other solubility rules for the AP, but it doesn’t hurt to be familiar with common soluble and insoluble compounds. Table 4.1 is a table of solubility for common ions in water. Usually, the question will tell you if the compound is soluble and which solution it’s soluble in😊.
Solubility Rules
Net Ionic Equations
We went over this in key topic 4.2, but let's do a quick🏃 overview!
The best steps to follow when writing a net ionic equation are:
- Figure out which compounds are soluble and insoluble using solubility rules
- Complete Ionic Equation - write insoluble compounds into ions
- Net Ionic Equation - cross out spectator ions and write the final result
Concentration of Ions
Knowing how to write the net ionic equation for a precipitation reaction is just the first step! Let's take a look at a concentration of ions question.
Question: 20.0mL of [0.100] NaCl(aq) reacts with 30.0mL of [0.0400] Pb(C2H3O2)2 (aq).
Part a: What is the mass of solid formed?
Part b: What are the concentrations of ions at the end of the reaction?
Step #1
Since they didn't give us the equation, let's write it ourselves!
NaCl + Pb(C2H3O2)2 --> NaC2H3O2 + PbCl2
💡Always check the equation is balanced! This one isn't, so let's balance it out and make sure each ion is in equal amounts on both sides.
2NaCl + Pb(C2H3O2)2 --> 2NaC2H3O2 + PbCl2
Step #2
With precipitation reactions and concentration of ions questions, there will always be an insoluble product. In this case, it is either NaC2H3O2 or PbCl2. Since sodium is always soluble, PbCl2 is the precipitate in this question.
NaCl (aq) + Pb(C2H3O2)2 (aq) --> 2NaC2H3O2 (aq) + PbCl2 (s)
Step #3
Now that we have the equation and know the precipitate, let's get into the math itself. The question provided us with the volumes and molarities for each reactant. Using these two pieces of information, we can find the number of moles of NaCl and Pb(C2H3O2)2.
Molarity = moles / volume in L - We have to convert the volumes we have into L by dividing by 1000.
0.100 = x moles of NaCl / 0.020 L --> x = 0.00200 moles of NaCl (aq)
0.0400 = x moles of Pb(C2H3O2)2 / 0.030 L --> x = 0.00120 moles of Pb(C2H3O2)2 (aq)
Step #4
Using the number of moles we solved for above, we can now use stoichiometry to answer part a.
But wait! Which number do we do stoich with, 0.00200 or 0.00120🤔?
This is where the limiting reactant (LR) comes into play. The limiting reactant in a reaction is the substance that limits the amount of products produced. Basically, there are different amounts of each reactant. One reactant is more abundant, right?
The reactant that there is less of eventually stops the reaction and limits it since the reactant runs out. The other reactant is called the excess since there is still some of it left over, unreacted.
To find the LR, we have to do stoichiometry with both amounts. Convert each reactant into the precipitate:
Since there are less moles of PbCl2 using NaCl as a reactant, NaCl is the LR. Pb(C2H3O2)2 is the excess.
Now we know how many moles of NaCl, Pb(C2H3O2)2, and PbCl2 we have and can answer a for real now!🥳
0.00100 mol PbCl2 x 278.2 g/mol = 0.278 g of PbCl2
Step #5
Yay, we did half the problem! Let's move on to solving for the concentrations of ions. In order for us to do this, we have to know the moles of each ion and the volumes of each ion.
Let's think this through conceptually a bit🧠. After PbCl2 (s) forms, what is left in the solution?
Looking back at the LR, either Na+ or Cl- will have a final concentration of 0 since they one of them will be completely used up. Since Cl- is in the precipitate, Cl- has a final concentration of 0.
That was easy! 1/4 of part b complete 😊.
The ion that is in the LR and precipitate ALWAYS has a final concentration of 0.
Step #6
In this next step, we can solve for the concentrations of two ions: Na+ and C2H3O2. These are considered the spectator ions since they aren't in the precipitate.
To find their concentrations, we have to use both the 0.00100 mol of PbCl2 from using NaCl and the 0.00120 mol of PCl2 from using Pb(C2H3O2)2.
The first number can help us find Na+ whereas the second can help us find C2H3O2-.
Na+: All you have to do now is find the volume, but we have to multiply the number of moles by 2 since NaCl has an initial coefficient of 2. To find the volume, we just have to add 20.0mL and 30.0mL.
(0.00100)(2) / 0.050 L = 0.0400 M of Na+
C2H3O2-: We have to multiply by 2 here as well since there was a subscript on the reactant side of the equation.
(0.00120)(2) / 0.050 L = 0.0480 M of C2H3O2-
Step #7
Last one: Pb+2
This is slightly harder to find, but with some practice, you got this😌!
To find the excess amount of lead, convert the LR to the soluble product. Here, we would convert 0.00200 moles of NaCl to find the moles reacted. Since there is a 1:2 mole ratio, 0.00100 moles reacted. Then we would subtract by the excess number of moles (found in step 4), which is 0.00120.
0.00120 - 0.00100 = 0.00020 moles of Pb+2 unreacted. Then we just divide by the volume so 0.00020 moles / 0.050 L = 0.0040 M of Pb+2.
Final Answers
Part a: 0.278 g of PbCl2
Part b: [Cl-] = 0
[Na+] = 0.0400
[C2H3O2-] = 0.0480
[Pb+2] = 0.0040
This is a very difficult question but once you practice and understand it conceptually, you'll begin to understand it. It is honestly a lot in one question and probably won't be tested like this. However, knowing it will strengthen your overall stoichiometry skills, so it doesn't hurt!🙃
🎥Watch: AP Chemistry - Precipitation Reaction
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.