Dalia Savy
A
Anika P
Dylan Black
AP Chemistry 🧪
269 resourcesSee Units
From electronic devices📱 to fizzy soda🥤, our world runs on chemical reactions. So what happens in chemical reactions? In a simplified definition, specific interactions with molecules result in the rearrangement of atoms to create new molecules. However, this magic✨ has restrictions, as will be discussed in the next sections.
What is a Chemical Reaction?
There are two types of changes in chemistry: physical changes and chemical changes.
A physical change is one that changes an object, but does not change the chemical structure. For example, you may boil water into steam, but H2O stays H2O💧 during that process. Similarly, shredding paper would be an example of a physical change. Paper may change from a piece to shreds, but it stays "paper"📄.
However, chemical changes, as the name implies, ends with a brand new product. For example, if you leave iron out for too long, it rusts. Rust is a chemical reaction between iron (Fe) and oxygen in the air (O2) to form rust (iron oxide, Fe2O3). Key marks of a chemical change are things like gas production♨️, color changes🔴🔵, odors👃, etc. Another example of a chemical change is mixing baking soda (NaHCO3) with vinegar (CH3COOH) to form carbon dioxide and other products. Chemical changes are the focus of AP Chemistry, and are described using chemical reactions.
🧠There are five main types of reactions: synthesis, decomposition, combustion, single replacement, and double replacement. While we will go over these in later sections, let’s do a brief overview for each type:
Synthesis Reactions
Synthesis reactions are the easiest type to understand. Essentially, a synthesis reaction sees two reactants fuse to form products in the form A + B --> AB, where A, B, and AB are arbitrary.
For example: 2Na + Cl2 → 2NaCl is a synthesis reaction, as it sees two reactants, Na and Cl2, become one product - NaCl.

The Synthesis of Sodium Chloride from Sodium Metal and Chlorine Gas
Decomposition Reactions
Decomposition reactions are the exact opposite - one reactant decomposes into 2 or more products. This can be represented by the general equation: AB → A + B. For example, hydrogen peroxide decomposes into hydrogen gas and water in the reaction 2H2O2 → 2H2O + H2.

The Elephant's Toothpaste: A violent reaction that is caused by a speeding up of the decomposition of hydrogen peroxide using a catalyst
Combustion Reactions
A combustion reaction is a special type of reaction involving organic molecules, which are molecules that are carbon based. A special class of organic molecules are called hydrocarbons, since they only have hydrogen and carbon. When in the presence of heat and oxygen, hydrocarbons combust, or burn, which is really just the energy being released from a chemical reaction!
This chemical reaction sees a hydrocarbon being combusted into oxygen and carbon dioxide. For example, CH4 + 2O2 → CO2 + 2H2O is the combustion of methane (CH4). This definition of combustion is why oxygen is necessary for fires to start.
Combustion reactions always follow this format: Hydrocarbon + Oxygen = Carbon Dioxide + Water
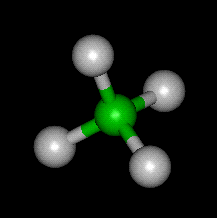
The Methane Molecule - Image Courtesy of world of molecules
Single Replacement Reactions
Single replacement reactions occur when you have a compound reacting with an element. The general form is AB + C --> AC + B. The most common form of a single replacement reaction is one called a redox reaction, where electrons are transferred between atoms. An example is 3Mg + 2AlCl3 --> 3MgCl2 + 2Al.
Single = one, replacement = an switch. One element switches places with another. Only one switch takes place.
Double Replacement Reactions
The most common type of reaction is a double replacement, with the general form AB + CD --> AC + BD. For example, if you mix an acid and a base, you get a reaction that forms water and a salt. A formula example would be HCl + NaOH -> NaCl + H2O. These reactions are the majority of the reactions you'll see in AP Chemistry, so get used to them!
Double = two, replacement = switch. Two elements switch places with another. Here, two switches take place.
Practice Questions
Identify what each of the following reactions are:
- Zn + 2HCl → ZnCl2 + H2
- 2C8H18 + 25O2 → 16CO2 + 18H2O
- 2H2O → 2H2 + O2
- AgNO3 + NaCl → AgCl + NaNO3
- 2Ni2O3 → 4Ni + 3O2
- 2Na + Cl2 → 2NaCl
- Cl2 + 2NaBr → 2NaCl + Br2
- BaCl2 + Na2SO4 → BaSO4 + 2 NaCl
- C3H8 + 5O2 → 3CO2 + 4H2O
Answers
- Single Replacement
- Combustion
- Decomposition
- Double Replacement
- Decomposition
- Synthesis
- Single Replacement
- Double Replacement
- Combustion
These reactions are just the basics. Once we dive deeper into the unit, we'll go over three important reactions:
- Precipitation Reactions
- Acid-Base Reactions
- Oxidation-Reduction Reactions
🎥 Watch: AP Chemistry - Introduction to Chemical Reactions
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.