8.6 Molecular Structures of Acids and Bases
3 min read•may 28, 2021
Dylan Black
AP Chemistry 🧪
269 resourcesSee Units
8.6: Molecular Structure of Acids and Bases
Describing Acid and Base Strength
This big idea is all about looking at the molecular structure (think lewis diagrams) of acids and bases and determining from such how strong an acid or a base is. We have learned in the past that there are strong acids and weak acids and strong bases and weak bases, but how can we compare these by simply looking at them? The first step in using molecular structures to make conclusions about strength is to understand how we can describe acids and bases as strong/weak based on their molecules itself.
This idea comes down to the stability of the conjugate base/acid of the acid/base in question. Remember that when describing the strength of a conjugate acid/base it is inversely proportional to the strength of the base/acid itself. That is to say that the stronger the acid, the weaker the conjugate base. That means that if our conjugate base is a very strong base, our acid is not very strong and vice versa. To know if a conjugate base is very strong we discuss the stability of the compound. The question we want to answer is essentially “will this compound attract an H+ ion?" for conjugate bases and 'will this compound readily donate an H+ ion' for conjugate acids.
Connecting Strength to Structure
Because we now understand how the strength of a conjugate base/acid relates to the strength of its parent compound, we're ready to move into looking at different structures. Let's start by looking at a few points about acid structures and strength.
One of the first ideas is that weaker bonds to an acidic H will lead to a stronger acid because that bond is more easily broken. For example, in halogenic hydrides (eg. HF, HI, HBr, HCl), the weakest H-X interactions occur with larger halogens. This means that as you move down the halogen group, the acids become stronger because larger atoms will give weaker interactions. Similarly, acid strength increases from left to right across a period and increases going down a group. This can actually help us understand why strong acids have very weak conjugate bases. When a strong acid like HCl dissociates, the conjugate base, Cl-, is incredibly stable. Cl- is a stable ion that will not readily react with much. This means that it is not a reactive base and therefore will create a more dissociative acid.

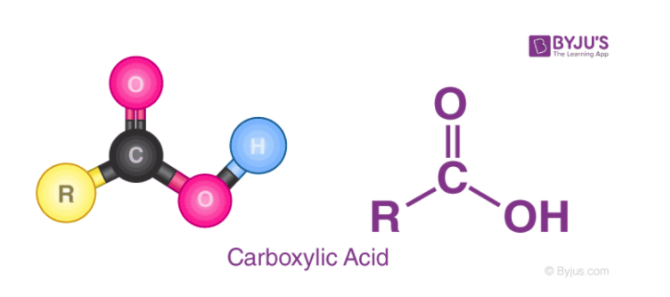
When discussing oxyacids, the structure can be described by looking at the polarity of the bond between the acidic oxygen (the oxygen attached to the acidic hydrogen). The easier it is for that O-H bond to break, the stronger the acid. In the following image, we represent the "rest" of the acid as "Z". The OH bond is easy to break when Z is either very electronegative or has a high oxidation state. An example of a class of weak acids that are oxyacids are carboxylic acids. These are acids that have a COOH group on the end such as CH3COOH. These acids are relatively weak because of the low electronegativity on the carbon making the bond less polar.

Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.