Dylan Black
AP Chemistry 🧪
269 resourcesSee Units
8.10: Buffer Capacity
Welcome to the last section of unit 8! This section is a relatively simple and short one all about buffer capacity which helps us gauge the effectiveness of buffers. For a quick reminder, buffers are important because they are resistant to changes in pH. However, they are not infinitely resistant. Eventually, the buffer will weaken and succumb to the acid/base being added. This is why, despite there being buffers in your bloodstream, chugging hydrochloric acid is a very bad idea. Seriously, don't do it. Buffer capacity helps us see how much acid/base one can add until there is a significant change in pH.
Describing Buffer Capacity
As said by the Henderson-Hasselbalch equation, the pH of a buffer is defined by the ratio of the concentrations of the conjugate base to the acid, or in math terms [A-]/[HA]. The capacity of a buffer is determined by the magnitudes of these concentrations.
Let's get into what we mean when we say magnitude. Essentially, the magnitude of each concentration describes how large the concentrations are. A concentration of 5M would have a higher magnitude than a 0.5M solution. The more concentrated the acid and conjugate base, the stronger the buffer is at reducing pH changes. This is because there is more acid and conjugate base to be resistant to strong acids/strong bases in a similar volume.
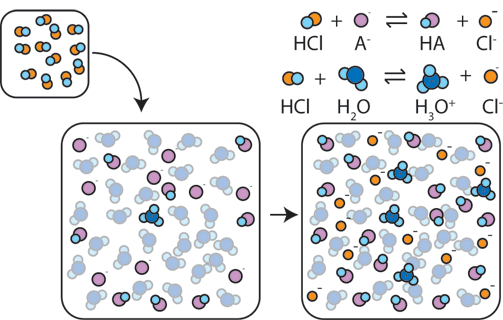
Image From ChemCollective
Example Problem: Identifying the Stronger Buffer
Let's think about two separate buffer systems, one with 5M acetic acid and 5M sodium acetate and another with 0.05M acetic acid and 0.05M sodium acetate. Because the ratios of the conjugate base to the acid are the same, both of these buffers will have the same pH (pH=4.74). However, our question asks us the following:
After HCl is added to each buffer system, the first one has a resulting pH of 4.74, and the second one has a resulting pH of 4.56. Which one has the better buffering capacity and why?
We can see that in the first buffer, the pH remained relatively unchanged whereas in the second the pH dropped. This means that the first buffer is more effective at resisting changes in pH and therefore has a better buffering capacity. This can also be explained by seeing that the magnitudes of the concentrations of both the acid and the conjugate base are higher in the first buffer compared to the second. This implies that the first buffer will have a stronger buffer capacity.
Practice Multiple Choice Questions
The CollegeBoard likes to use buffer capacity specifically on multiple choice questions because unlike many other questions relating to buffers, questions about buffer capacity are very often qualitative. This means that your answer will relate to some sort of non-numerical conclusion based on information you’re given. They could ask for the stronger buffer like in the last question or ask about a change in a system and how this may affect the buffer capacity. Take a look at this question:
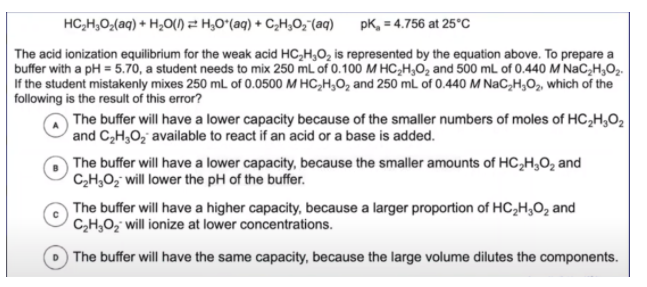
This problem gives us a BUNCH of information and can be really overwhelming at first but if we break it down piece by piece we’ll find that the problem is actually really approachable. We’re told that a student is creating a buffer of acetic acid (CH3COOH or also stated as HC2H3O2) and sodium acetate. We know this is a buffer because we have a weak acid mixed with its conjugate base. Let’s take a look now at the numbers we’re given. The first set of numbers tells us that the student wants to mix 250 mL of 0.100M acetic acid with 500 mL of 0.440M sodium acetate. Next, we’re given similar numbers but with an error. The student instead chooses an acid with half the concentration and uses half the volume of the conjugate base. We know this because we can compare the second set of numbers to the first and see that for acetic acid the volume remains at 250mL but the molarite is now 0.0500M and for sodium acetate the molarity stays at 0.440M but the volume falls from 500mL to 250mL. The problem finally asks which of the following choices is a result of this error.
Before looking at the answer choices, let’s just think about what has changed. For both sets (the weak acid and the conjugate base) the number of moles has halved. For CH3COOH the concentration has halved whereas for sodium acetate the volume has been cut in half. Therefore we will have half the number of moles of each species in our buffer. Again before looking at any answer choices let’s think about how this impacts the buffer. Like we’ve discussed, with a lower number of moles of each reactant in the buffer there will be less resistance to changes in pH. In other words, the buffer capacity has lowered. Let’s now take a look back at our answer choices and see if anything matches that idea:

Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.