Jacob Jeffries
AP Chemistry 🧪
269 resourcesSee Units
Types of Acids and Bases
Arrhenius Definition
There are two main schools of thought for what should be the definitions for acids and bases. The Arrhenius definition of an acid is any compound that increases the concentration of hydrogen ions ([H+]) in a solution, while the Arrhenius definition of a base is any compound that increases the concentration of hydroxide ions ([OH-]) in solution. Essentially, the Arrhenius definition of an acid/base is anything that yields H+ or OH- respectively in water. For example, HCl (hydrochloric acid) can be described as an Arrhenius acid because in water the following reaction occurs:
HCl → H+ + Cl-
Thus, HCl yields an H+ ion in water, making it an Arrhenius acid.
Brønsted-Lowry Definition
Meanwhile, the Brønsted-Lowry definition of acids and bases define acids and bases in the form of a donation reaction. Basically, an acid/base is seen rather as an H+ donator or accepter (the acid donates, the base accepts):
HA + B- → HB + A-
In this example, HA is the acid, which donates an H+ ion to the B- ion (the base) to form HB and A-. Quick tip, get used to seeing HA and B- as sample acids and bases, it's just notation. A and B could be standins for any number of ions that fit in. Quick thinkers may be wondering about the reverse reaction in the case that HA + B- is an equilibrium reaction:
HA + B- ⇄ HB + A-

The Hydronium Ion
A consequence of Bronsted Acids and Bases is that when an acid dissolves in water, it needs something to donate it's H+ to! In this case, it donates it to water, creating H3O+, or hydronium. Thus, we can see the dissolution of an acid both in an Arrhenius sense as HA <--> H+ + A- and a Bronsted sense as HA + H2O <--> H3O+ + A-. Both of these essentially mean the same thing (and when we learn pH, we'll find that [H+] = [H3O+]), but they display the difference between Arrhenius and Bronsted Acids.
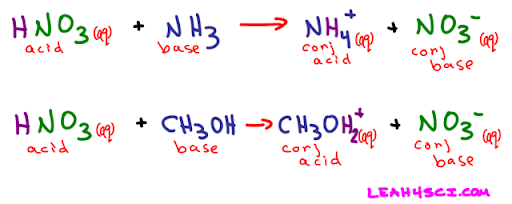
Conjugate Acids and Bases
How do we classify HB and A- then? Wouldn’t A- be a base and HB be an acid if the reaction were reversed? The answer is short: yes. However, they are given a special name: HB is called the conjugate acid of B-, and A- is called the conjugate base of HA. In the case that HA is a weak acid and B- is a weak base, HB and A- would be considered to have significant acidity/basicity. The conjugate acid/base of a strong base/acid does not have acidity/basicity. The weaker the acid/base, the stronger the conjugate base/acid.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.