Jacob Jeffries
AP Chemistry 🧪
269 resourcesSee Units
What are Acids and Bases?
The general population has a vague idea of what an acid is; this is the same case for bases, albeit the idea for bases is a little less common. Personally, as a child, whenever I had heard the word “acidic” I immediately thought of a lemon. Now the case is a little different; I actually think of the chemical definition when I hear the word because organic chemistry took away my childhood innocence.
Why Do Acids and Bases Matter?
Jokes aside, acid-base chemistry is the formalization of this concept, and is extremely applicable to the sciences, along with everyday life. If you drink sodas, which are acidic, acid-base chemistry is fundamental in precisely describing why it is unhealthy for your teeth.
If you do not drink soda, acid-base chemistry is essential to organic chemistry, and consequently medicinal chemistry. If it were not for modern science’s knowledge of acid-base chemistry, there would simply not be effective medicine for the general population to take.
Though, on the same note, acid-base chemistry describes why some darker sodas can be used as a cleaning product while still being digestible by humans.
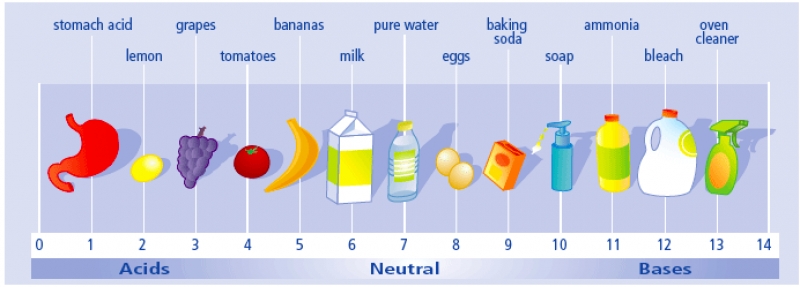
The pH scale, something you will be very familiar with by the time you finish unit 8.
What Will You Learn in Unit 8?
Acid-base chemistry essentially analyzes the complicated paths that free protons take whenever a chemical is dissolved in solution. A free proton is a bonded hydrogen that is accessible by other chemicals, whereas a restricted proton is a proton that is guarded by other atoms in a particular compound. In this unit, you'll look at measuring concentrations of free protons (H+ ions) to find pH and pOH regarding weak acids and bases that do not dissociate fully (meaning that the equilibrium that you learned last unit will come in handy!) and strong acids and bases that do.
Once you understand pH and pOH, you'll start moving into more complicated acid base chemistry like learning about buffers (mixtures of acids and conjugate bases of vice versa) and titrations, which you may be familiar with from unit 4. With titrations in particular, we'll be looking at more complicated ways to do titrations and make calculations based on data from such which is a big topic on the AP exam!
Acid Base Reactions are essentially the basis for any biochemical reaction, ie. it allows life to prosper, and there are only a select handful of things more important to human beings than our own lives. The exceptions to this are money and chicken tenders, which oddly enough also rely on organic chemistry to even exist. Now get excited, unit 8 is a hard unit, but also a suuuper interesting one!

Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.