Dylan Black
AP Chemistry 🧪
269 resourcesSee Units
9.5: Free Energy and Equilibrium
In unit 7 we spent a ton of time discussing the concept of equilibrium. Equilibrium helped us determine how a reversible reaction may behave under certain circumstances. In this section, we’ll look at how equilibrium connects to spontaneity and ΔG° by observing various definitions of equilibrium along with relationships between ΔG° (ΔG at standard conditions) and ΔG (ΔG at any other conditions).
Kinetic and Thermodynamic Definition of Equilibrium
When learning about equilibrium, the typical definition that we discussed is known as the kinetic definition of equilibrium. This definition concerns the rates of the forward and reverse reactions as the reaction approaches equilibrium. Recall that we defined equilibrium as the point at which the forward reaction and reverse reaction proceeded at the same rate meaning the concentrations of products and reactants stay the same. It’s important to note that equilibrium does not mean nothing is happening, just that both reactions are happening at the same rate, meaning they “cancel” each other out.
However, there is another important definition of equilibrium that concerns thermodynamics (the thermodynamic definition). This definition defines equilibrium as the point of minimum free energy. This is because while the reaction is spontaneously occurring, ΔG (note the lack of a naught (the ° next to G)) will be less than zero, meaning the reaction will be releasing free energy, and after it reaches equilibrium concentrations, ΔG will be positive and thus requires external sources of energy to occur (this can occur and we’ll discuss this in the next section). We can see this point visually in the following graphs in which the y-axis is free energy (G) and the x-axis is the extent of reaction from 100% reactants to 100% products:
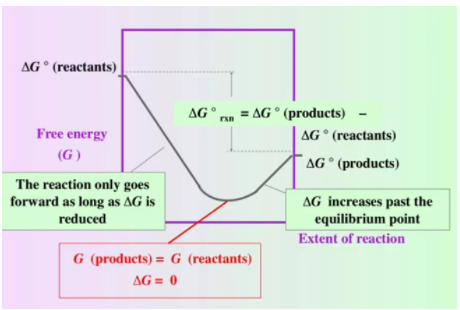
Whoa. What’s going on here? Let’s break this down piece by piece. First note that ΔG is not the y position of the graph but rather the rate of change of the graph. When ΔG < 0 the graph decreases and when ΔG > 0 the graph increases.
We begin on the far left with 100% reactants and so ΔG = ΔG° of the reactants. Similarly, on the far right, we have 100% products and so ΔG = ΔG° of the products. We can see that the ΔG° of the products is lower than the ΔG° of the reactants meaning that ΔG° for this particular reaction is negative and thus the reaction is spontaneous. This is because ΔG° = ΣnΔG°f (products) - ΣnΔG°f (reactants).
For this reaction, we begin with a negative ΔG. This means the reaction is still in a spontaneous “state” and therefore we will continue producing products. We move forward until we hit the point at which ΔG=0. This is the minimum point of free energy. From what we’ve discussed, we know that this point is the equilibrium point. From this point forward ΔG > 0 and thus, we need to add energy to the system to produce more product. This is because the reaction is nonspontaneous.
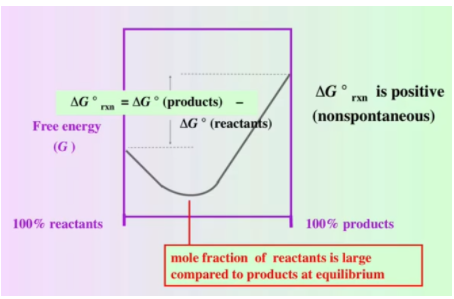
The above graph is the same as our graph from before except note that our ΔG° (products) is now greater than ΔG° (reactants) and thus ΔG° is positive for this reaction. This tells us that the reaction is nonspontaneous. We’re also told that the mole fraction of reactants is large compared to products at equilibrium. This means that we have many more reactants than products. This is because, as we’ll see in a bit, a nonspontaneous reaction is linked to a reactant factored reaction.
Relationship Between ΔG°, ΔG, and K
Now that we’ve looked at a qualitative relationship between ΔG°, ΔG, and K, let’s look at some of the mathematical equations that are used to describe this relationship. Remember that ΔG is a measure of free energy change at nonstandard conditions, so a relationship can be drawn between ΔG° and ΔG by connecting it to Q, the reaction quotient:

Remember that to calculate Q; we plug non-equilibrium concentrations or pressures in the law of mass action (Our equilibrium formula). R and T are the gas constant (8.314 J/molK or whatever fits your units) and T is the temperature in Kelvin.
Next, we can look at the relationship between ΔG° and K. Using our above equation, we can derive a relationship directly with only ΔG°. At equilibrium, we have two main conditions: ΔG = 0 and Q = K. Substituting into our equation we find the following:
0 = ΔG° + RTln(K)
ΔG° = -RTln(K)
Solving for K:
-ΔG° = RTln(K)
-ΔG°/RT = ln(K)
K = e^(-ΔG°/RT)
These two equations show a direct relationship between ΔG° and K. Qualitatively, we can see that a higher ΔG° means a lower K and vice versa. With a positive ΔG° we see that -ΔG°/RT is negative and therefore K is e^(negative number) meaning it is less than 1. Similarly with a negative ΔG°, -ΔG°/RT is positive and so K = e^(positive number) and is thus greater than 1.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.