Dylan Black
AP Chemistry 🧪
269 resourcesSee Units
9.4: Thermodynamic and Kinetic Control
In this section, we’ll take a look at the relationship between thermodynamic favorability and kinetics, specifically in the shortcomings of Gibbs Free Energy in predicting reaction behavior. We’ll see that many reactions, despite being spontaneous, do not occur at an observable rate. This is because many reactions are under what we call kinetic control. In this guide, we’ll explore what kinetic control means and why it occurs.
Brief Review of Kinetics
Before getting into the thermodynamics of kinetic control, let’s take a quick look back to unit 5 and some topics from kinetics that we’ll need to understand kinetic control. If you’re confident in your kinetics chops, feel free to skip this section. Recall that kinetics is the study of the rate of a reaction - essentially how fast a reaction occurs. We measure the rate of a reaction by measuring the change in the concentration of reactants over time. Your teacher may have written this as R = Δ[A]/Δt or if you know calculus, you may have seen it as R = -d[A]/dt. The higher this number, the quicker we are losing reactants and forming products. Rates can also be described using rate laws in which initial reactant concentration is directly tied to the rate of a reaction as follows: R = k[A]^n[B]^m… where [A]^n, [B]^m, etc are various reactants raised to their reaction order - that is how much of an impact their concentration has on the rate. Take a look at the following rate law as an example:
rate = k[A]
These laws help us describe how quickly a reaction will occur, with a higher rate of course implying a faster reaction overall.
Most important to our study of kinetic control is activation energy. Recall that due to the kinetic molecular theory, chemical reactions occur due to molecules hitting each other at the right angle and speed/energy. This is the activation energy and is the energy required for a chemical reaction to actually occur. The higher the activation energy, the harder it is for the reaction to occur at an observable rate. The following diagram visually shows the concept of activation energy:
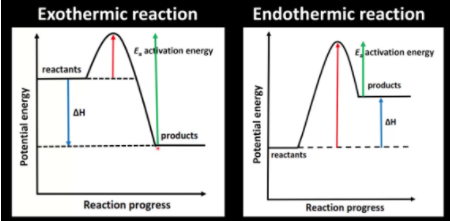
Image From Mike Sugiyama Jones
A Shortcoming of Gibbs Free Energy
A common misconception when looking at thermodynamic favorability is that a thermodynamically favorable reaction occurs quickly. Many reactions that are spontaneous in fact can occur incredibly slowly. A good example of this concept that can be applied to the real world is the conversion from diamonds into graphite (represented as Cdiamond(s) → Cgraphite(s)). For this reaction, ΔG° = -3 kJ. This tells us that the reaction occurs spontaneously and thus doesn’t require the input of any external energy to occur. However, take a look at the nearest diamond (because people have those around the house…right?). Is it suddenly morphing into graphite? No! (I hope not. Fiveable is not responsible for diamond graphitification…). This is because the conversion of diamond into graphite is incredibly slow. Like, thousands of years slow. We say that this reaction is in kinetic control because it is driven by the slowness of the reaction. These types of reactions are often slow because they have a high activation energy.
A spontaneous process may take either the thermodynamically controlled or the kinetic controlled pathway. In a kinetic controlled path is like the one above and is driven by a high activation energy. A thermodynamically controlled reaction is driven by the difference in free energy between the products and reactants, the type of reaction we saw in the last section.
Reasons For Kinetic Control
As we mentioned before, the primary reason for a reaction to be under kinetic control is because of a high activation energy. Because of this, even if the reaction is thermodynamically favorable, it may not continue at a measurable rate. There is a way around this, however, and that is through the use of a catalyst! Catalysts change the mechanism behind a reaction in order to decrease the activation energy and make reactions quicker. By reducing the activation energy, the reaction can proceed at a measurable rate.
For example, the decomposition of hydrogen peroxide usually occurs at an unmeasurable rate. However, when iodide ions are used as a catalyst, it creates the famous elephant’s toothpaste reaction which you may be familiar with.

This example shows us how catalysts can transform a reaction from one at an immeasurable rate to one at a measurable rate. This can be applied to kinetic control by noticing that these catalysts can help get a reaction out of kinetic control by lowering the activation energy. For example, if there was a catalyst for the reaction Cdiamond(s) → Cgraphite(s), the reaction would be able to proceed at a measurable rate. However, without one, it cannot because the rate of the reaction is incredibly slow.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.