5.6 Reaction Energy and Graphs w/ Energy
2 min read•january 13, 2023
Dylan Black
AP Chemistry 🧪
269 resourcesSee Units
🎥 Watch: Activation Energy
Energy In Reactions
Graphs of Energy w/ Respect to Reaction Progress
As you have probably seen, potential energy in a reaction can be represented as a curve with a hump as the reaction progresses, with energy changes being able to be seen regarding the reaction. Typically, these are used to tell us if a reaction is endothermic or exothermic, that is to say, is the system gaining energy or losing energy? Here's an example:
.png?alt=media&token=5a9fcca7-a6e7-40db-a6c1-67b3f8f492ef)
Image Courtesy of the University of Illinois
You can ignore "activation energy" for now, we'll get into that in the next section. What's important for you to understand
is that in a reaction, energy is either released or absorbed and this will affect the energy involved in getting the reaction started.
is that in a reaction, energy is either released or absorbed and this will affect the energy involved in getting the reaction started.
The Progress of a Reaction
There are three main parts of a reaction: the reactants, the activated complex, the products. You've probably heard of reactants and products before, those being the stuff that goes in and comes out. However, the activated complex is the middle. Essentially, it's where a reaction is transitioning from reactants to products, and where bonds are not completely broken:
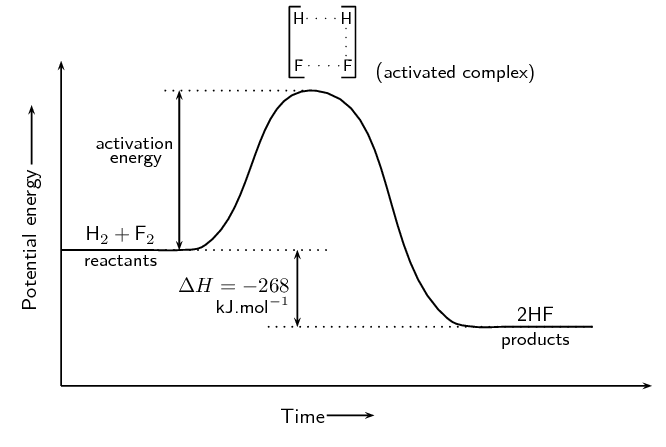
Activation Energy and the Arrhenius Equation
What is Activation Energy?
Activation energy is actually quite simple - it is the energy required to break the bonds in a reaction to go from the reactants, to the activated complex, to the products. It is defined formally as "the energy difference between the reactants and the transition state" according to the College Board. On an energy diagram, this is shown by an arrow from the reactants to the peak of the graph, as you can see in the prior images.
The Arrhenius Equation
The Arrhenius equation relates the temperature dependence of the rate of an elementary reaction to the activation energy needed by molecular collisions to reach the transition state:
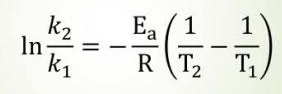
This equation can tell us how a rate constant changes based on changes in temperature. Note that for the AP exam, you will not have to use this equation to make calculations.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.