Dalia Savy
Dylan Black
AP Chemistry 🧪
269 resourcesSee Units
What is Kinetics?
Welcome to the first part of unit 5! This unit will cover everything you need to know about kinetics or the study of the rate of a reaction. Essentially, kinetics studies what makes reactions happen and how quickly reactions occur. You may notice that when doing labs or observing reactions, some reactions go incredibly quickly, whereas others go unbearably slow.
For example, if you take a balloon, fill it with methane, and put a match to it, the balloon explodes, whereas, with something like hydrogen peroxide, it breaks down incredibly slowly. The role of kinetics is to help describe why certain reactions are faster than others.
How Do We Measure the Rate of a Reaction?
The rate of a reaction (also shorthanded as "rate of reaction") has a simple definition that when uncovered has a lot of nuance to it. The simple definition of the rate of reaction is how quickly a reaction produces products. However, this definition brings up some issues. How do we measure "how quickly" a reaction occurs? Well, we do this by observing concentrations.
As a reaction progresses, the concentration of the reactants decreases as they are used to create products. A reaction may start with an initial concentration of 0.5M but then after 30 seconds, that concentration may drop to 0.2M. Conversely, the concentration of the products will increase as they are created. Therefore, when dealing with a rate of reaction, we can think about the rate at which the amount of reactants is converted to products in a certain period of time.
The rate of reaction can be written mathematically as Rate = -Δ[Reactant]/t or as Rate = Δ[Product]/t. The units for rate are mol/Ls, also notated as Ms^-1 or mol L^-1 s^-1. You may also see that seconds will change to hours, etc. You have to be sure to keep track of this and ensure your units are correct when doing math!
Showing Rate of Reaction Graphically
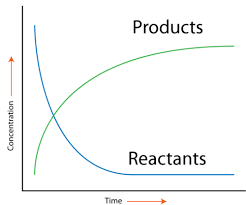
Image Courtesy of CK-12
As a reaction progresses, the [products] increases whereas the [reactants] decreases until the reaction reaches equilibrium. You can think of the equilibrium of a chemical reaction in the same way as homeostasis for our bodies. Both involve the maintenance of stability and balance in a closed system.
Equilibrium is defined as the point at which the rate of the reaction going forwards is the same as the rate of the reaction going backward and the concentrations of the reactants and products remain constant. This is covered in-depth in unit seven, but it's good to get a taste of it to understand this graph. Graphically, rate is the slope of the line between two points on either curve. This is because the slope of a line represents the change in concentration over the change in t, which as we discussed, gives us the rate! We can see this either as an average rate or an instantaneous rate.
The average rate of a reaction is the change in concentration of a reactant or product over a specific time interval (aka between two points). This is typically calculated by dividing the change in concentration by the time interval over which the change occurred. The average rate can vary over time, depending on the concentrations of the reactants and products and the conditions of the reaction.
The instantaneous rate of a reaction, on the other hand, is the rate of the reaction at a specific point in time. It is calculated by taking the limit of the average rate as the time interval approaches zero. This is equivalent to finding the slope of the line tangent to any given point, so you do need some calculus (which is beyond the scope of this course). For calculus people, you should recognize this as a derivative (Rate = -d[R]/dt).
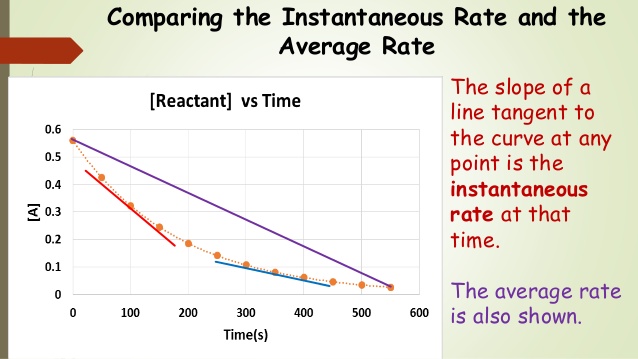
Overall, the main difference between the average rate and the instantaneous rate is that the average rate is a measure of the change in concentration over a specific time interval, while the instantaneous rate is a measure of the rate of change of concentration at a specific point in time.
Using Stoichiometry with Rate of Reaction
Let's suppose we had the reaction 2A + 3B → C. This reaction is, of course, imaginary, but using examples like this helps us simplify concepts instead of jumping straight into the crazy chemistry.
Let's suppose that in 2 seconds, [A] decreased 0.2M. Therefore, the rate of the reaction in terms of A is -0.2/2 = -0.1 mol A/Ls. Using this information, let's figure out the rate at which B is being used up. We can do this with some simple stoichiometry:
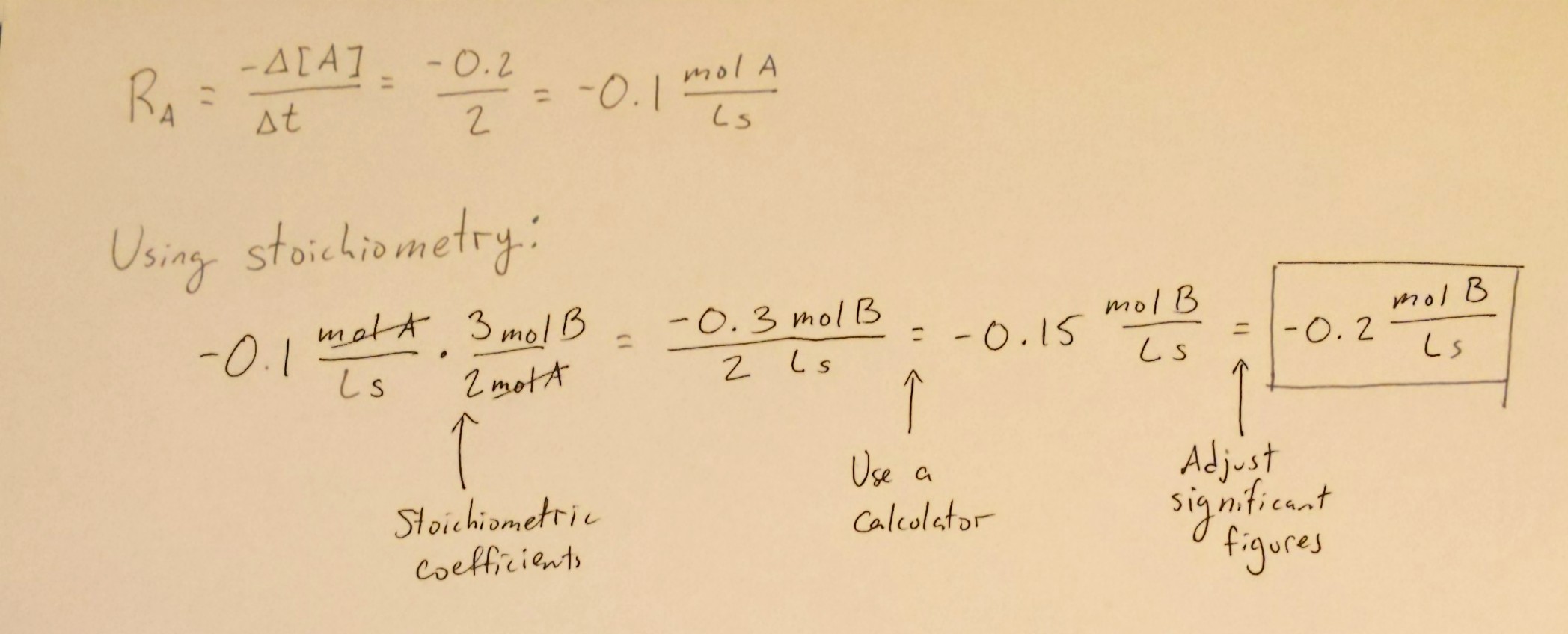
The same can be applied to C to find the rate of production of C. Stoichiometry continues to be a big tool in chemistry, and you can use it in this unit when calculating the rates of change of reactant and product concentrations.
Physical Attributes and Reaction Rate
There are several physical attributes that can influence the rate of a chemical reaction. These include:
- Concentration: Increasing the concentration of reactants generally increases the rate of a reaction. This is because a higher concentration of reactants means that there are more reactant molecules present, increasing the chances of successful collisions between reactant molecules.
- Temperature: Increasing the temperature of reactants generally increases the rate of a reaction. This is because an increase in temperature means that the reactant molecules have more kinetic energy, which increases the chances of successful collisions between reactant molecules. Remembering that temperature is the average kinetic energy is key to this unit.
- Surface area: Increasing the surface area of a reactant (e.g. by grinding a solid reactant into a powder) can increase the rate of a reaction. This is because a larger surface area means that there is more surface available for reactant molecules to collide with, increasing the chances of successful collisions.
- Presence of a catalyst: A catalyst is a substance that increases the rate of a reaction without being consumed by the reaction. Catalysts work by providing an alternative pathway for the reaction to occur, which can lower the activation energy needed for the reaction to take place. We'll come back to this concept later in this unit.
- Pressure: For reactions involving gases, increasing the pressure can increase the rate of the reaction. This is because a higher pressure means that there are more gas molecules present in a given volume, increasing the chances of successful collisions between reactant molecules.
If you ever have trouble trying to understand these relationships, review those between temperature and pressure from unit three! Believe it or not, gas laws help your understanding of kinetics quite a bit.
.gif?alt=media&token=0ff96e5b-5811-483f-9336-12666c0e27dd)
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
🧐Exam Skills
📚Study Tools

Fiveable
Resources
© 2025 Fiveable Inc. All rights reserved.