5.7 Reaction Mechanisms and Elementary Steps
3 min read•january 13, 2023
Jacob Jeffries
AP Chemistry 🧪
269 resourcesSee Units
What is a Mechanism?
Reaction mechanisms go more in-depth regarding chemical reactions than just a net equation. For example, a reaction between an acid and a base must happen in the presence of water, yet water is not included as a reactant. The actual reaction happens in two steps. A mechanism takes a full reaction and splits it up into elementary steps, essentially what actually happens in a reaction. Here's an example for the decomposition of hydrogen peroxide (H2O2):
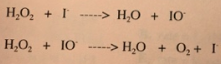
Catalysts and Intermediates
As you can see, each of these steps are individual reactions, and when added up and spectators are cancelled out, we end up with the total reaction. Why is this important, you may ask? Well, if we find the total reaction by adding the elementary steps, we see that IO- and I- cancel out to leave 2H2O2 --> 2H2O + O2. So then why are they there? Well, as we'll see in future lessons, iodide acts as a catalyst for this reaction, speeding it up. It goes in and comes out the exact same way and thus does not impact the reaction products, but rather impacts the mechanism by changing the way the reaction occurs. The natural decomposition of H2O2 actually occurs, but it is incredibly slow. By changing the steps in the reaction, iodide creates IO-, which is called an intermediate. It is not a product, nor a reactant, but takes place in the reaction.
What a mechanism looks like for a more complex reaction. Luckily you won't be dealing with these! Courtesy of ResearchGate
Reaction Mechanisms and Rate Laws
Rate Determining Steps
Often when dealing with mechanisms, you will see one elementary step labeled "slow" and the others labelled "fast". The slow step is also called the rate-determining step as it defines the rate law. Let's take a look at an example:

Example Courtesy of the Organic Chemistry Tutor
Step one is to find the overall reaction for this mechanism, which comes out to H2 + 2ICl --> I2 + 2HCl. We now know what the overall reaction is. To find the rate law for this reaction, we must look at the rate limiting step. When dealing with elementary steps (AND ONLY ELEMENTARY STEPS), we can use the stoichiometric coefficients to tell us the order for each reactant. Thus, looking at the slow reaction, we know that the rate law is: R = k[H2][ICl]
When the Slow Step Has Intermediates
An important thing to note is that sometimes the rate-determining step will have an intermediate in it. In this case, you will need to use some math to make a substitution since you cannot have an intermediate in your rate law. This math involves a topic in chemistry that you most likely learned called equilibrium. If you have, what you do is you essentially use the Keq of one of the elementary steps (typically one will be in "fast equilibrium") and use some substitutions. To see what this looks like, check out the 2019 Scoring Guidelines, Page 14.
Example Mechanism
We will consider the following mechanism:
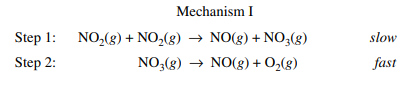
Example Courtesy of CollegeBoard
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.