6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium
2 min read•january 13, 2023
Dalia Savy
A
Anika P
AP Chemistry 🧪
269 resourcesSee Units
Molecular Collisions
Temperature reflects the average kinetic energy, or random motion, of particles. Kinetic energy is speed, so as temperature increases, molecules collide with the walls of the container faster.
The Collision Theory
Collision theory is based on the following postulates:
- The rate of a reaction is proportional to the rate of the collisions 💨
- Orientation matters for the reactant molecules colliding ➡️⬇️
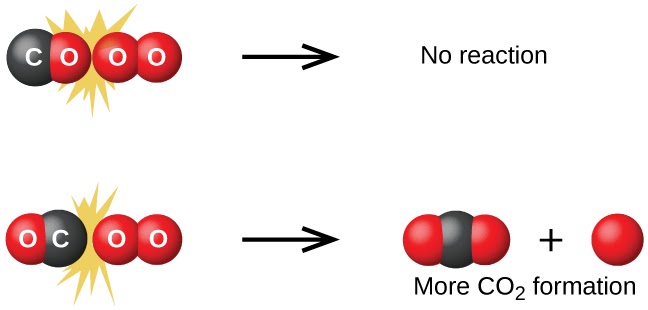
Image Courtesy of Chemistry BC Textbook
Successful collisions would therefore collide with proper orientation to break bonds and have enough energy to overcome the reaction’s activation energy.
Just remember, proper orientation + enough energy = successful collision.
If we look at this in terms of heat transfer, higher temperatures mean a higher number of collisions because the reactants are moving quickly. Heat conduction involves this high temperature state going to a low temperature state, where collisions are not as frequent due to the decrease in temperature.
Transfer of Heat Between Objects
As we see in real life, heat travels from a hot object to a cold object until the two are in thermodynamic equilibrium (thermal equilibrium). Take for example, putting a pan🍳 up against hot grates on a stove that are heated up by a fire. The heat travels from the grates to the pan until the two are the same temperature.
Basically, the heat goes from the source (hot item) to the sink (cold item), until thermal equilibrium is reached. At thermal equilibrium, molecules are moving at the same speed since the temperatures are the same.
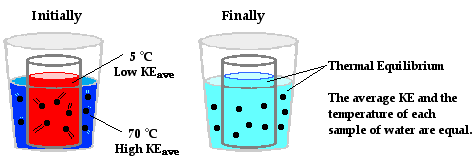
Image Courtesy of ThePhysicsClassroom
The Zeroth Law of Thermodynamics
The Zeroth Law of Thermodynamics is stated as "if a is in thermal equil. with b and b is in thermal equil. with c, then a is in thermal equilibrium with c" where a, b, and c are bodies.
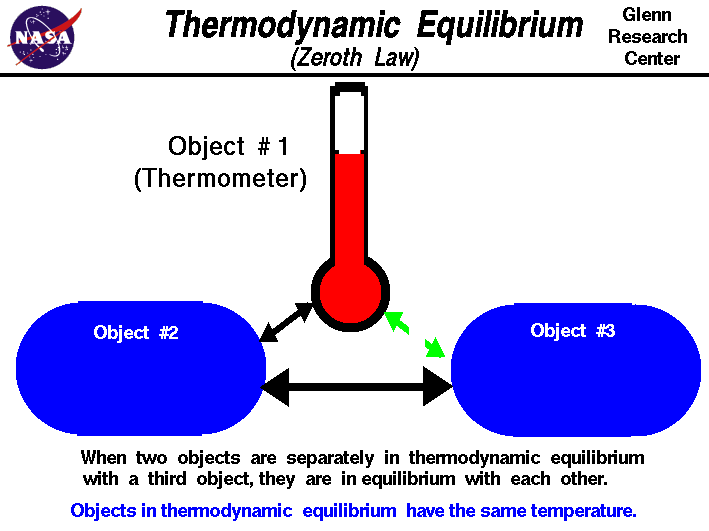
Image Courtesy of NASA
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.