6.6 Introduction to Enthalpy of Reaction
3 min read•january 13, 2023
Dalia Savy
A
Anika P
AP Chemistry 🧪
269 resourcesSee Units
Review of What Enthalpy Is
Enthalpy is a thermodynamic quantity equivalent to the total heat content of a system. Basically, enthalpy helps describe heat energy within a system and how this heat energy moves through, in, and out of a system🔄. However, as we've previously discussed, you cannot measure absolute enthalpy, but rather you can only measure changes in enthalpy, or ΔH.
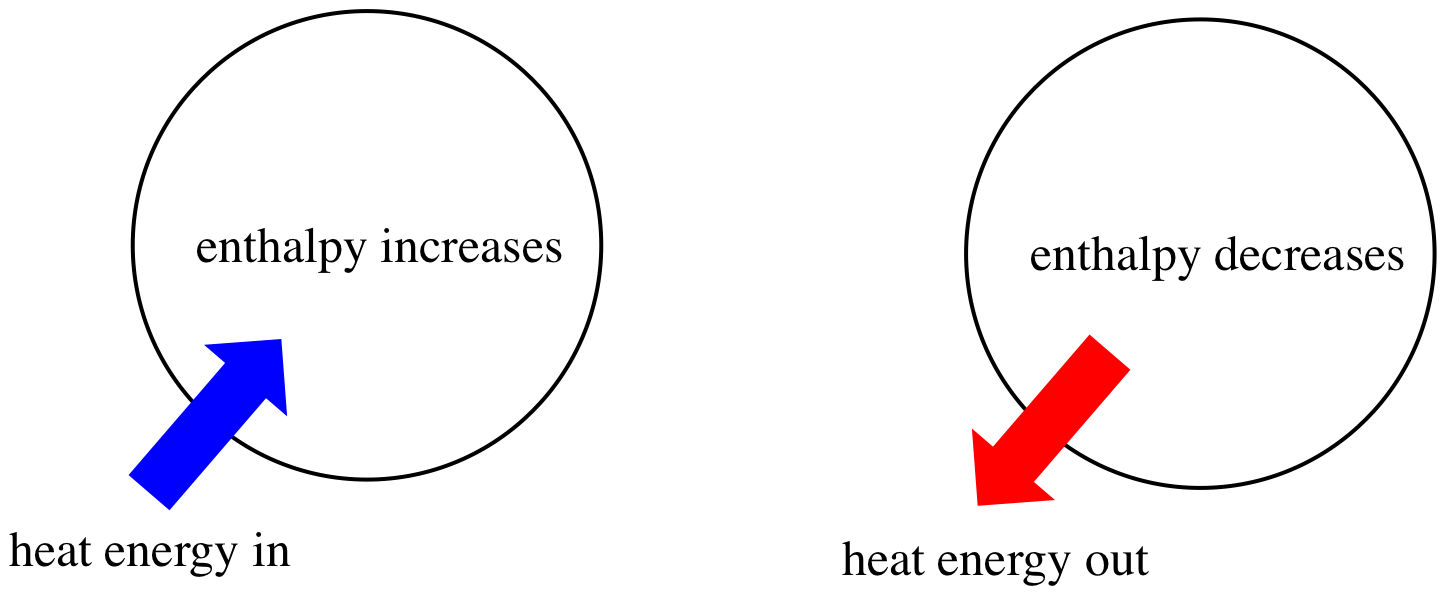
Image Courtesy of Chemistry LibreTexts
Reaction Energy, AKA: Enthalpy of Reaction
In Unit 6.1, we identified two types of reactions: exothermic and endothermic reactions. You may think of an exothermic reaction as "hot" or "heat producing" and an endothermic reaction as "cold" or "heat absorbing". While you can think of these reactions in terms of temperature🌡️ or in terms of the change in enthalpy.
The change in enthalpy due to a reaction is called the enthalpy of reaction and describes whether a system is losing or gaining energy (at constant pressure). If ΔH is positive, the system is gaining energy, and if ΔH is negative, the system is losing energy🔥.
Negative ΔH vs. Positive ΔH
Now that we've learned about what enthalpy of reaction means, let's look at the difference between the signs of ΔH (+ or -). If ΔH for a reaction is positive, the system is gaining energy, meaning the reaction is endothermic. This might seem counterintuitive, but since the surroundings is losing energy, we will see the reaction cooling down the environment, which is our observational definition of endothermic.
Similarly, if a reaction's ΔH is negative, the reaction is exothermic, since energy is being released into the surroundings (the system is losing heat).
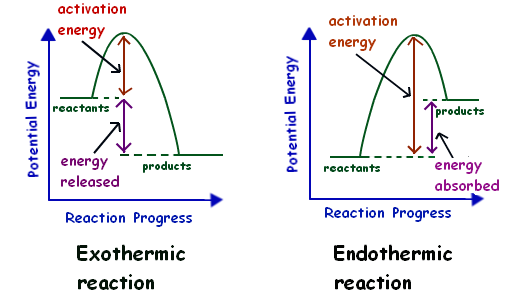
Image Courtesy of Mr Lowe
Other Terms to be Familiar with
Energy, enthalpy, and heat, what is the difference between these things? And what is work?
As we went over before, energy is generally the capacity to do work or produce heat.
Internal energy⚡, which is referred to as E sometimes, is the sum of the kinetic energy in a system and the potential energy in a system. You could calculate the change in E, or ΔE, using the following formula: ΔE = q + w.
Heat🕯️, or q, refers to the transfer of energy between two objects that occurs due to a temperature change. Remember, heat flows from the source, which is the hotter substance, to the sink, which is the cooler substance.
Work🏃, or w, refers to force over a distance. Work is really a physics term, but know that if you are gaining work, or have positive work, a gas is compressed, volume decreases, and E increases. Vice versa applies as well: if you are losing work, or have negative work, a gas is expanded, volume increases, and E decreases. You don't really have to know this but w=-PΔV. Work is usually expressed in joules. Basically:
- Positive work = compression = volume decreases = energy of the system increases
- Negative work = expansion = volume increases = energy of the system decreases
On the AP Chemistry exam, enthalpy and internal energy are synonymous terms. Same goes with bond energy and bond enthalpy, which we will review in the next key topic!
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.