6.1 Endothermic Processes vs. Exothermic Processes
4 min read•january 13, 2023
Dalia Savy
A
Anika P
AP Chemistry 🧪
269 resourcesSee Units
Forms of Energy
Matter can undergo physical and chemical changes. Such energy changes are related to temperature changes🌡️. This can easily be seen with the melting of ice:
H2O (s) → H2O (l) Energy is supplied to melt the ice.
Energy is the capacity to do work or transfer heat🔥. There are three types of energy that are commonly tested:
Kinetic Energy
Kinetic energy is the energy that results from the motion of an object.
KE=½mv^2, where mass, m, is expressed in kilograms and velocity, v, is expressed in meters per second.
KE is expressed in Joules. You don't have to memorize this formula; just be aware that mass and velocity impact the kinetic energy of an object.
Potential Energy
Potential energy is the stored energy in an object based on its position. It usually results from both attractive and repulsive forces.
Electrostatic Energy
Electrostatic energy is a form of potential energy that results from the interaction of charged particles. You could associate this with Coulomb's Law, which we discussed several times in previous chapters.
PE of an electron = Q1Q2/d, where charges, Q1 and Q2, are separated by a distance, d
Remember, attraction between ions only occurs if the charges are opposite and repulsion only occurs if the charges are the same.
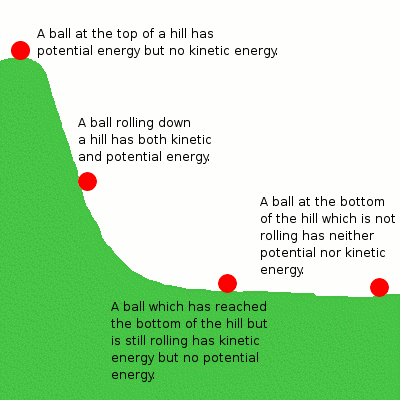
Image Courtesy of NRG
The Law of Conservation of Energy
This unit is based around one central idea: The Law of Conservation of Energy.
Since it is so so important, it is also called the First Law of Thermodynamics. It states the total amount of energy in the whole universe is constant. Therefore, energy can be neither created nor destroyed!
Studying Energy Changes
The System
To analyze energy changes, we first have to be able to define the system. This is a specific part of the universe that is of interest to us and would typically involve substances used in experiments (ex. HCl, NaOH, etc.). There are several types of systems:
- Open System ⚖- An open system always allows air, heat, and energy outside of it. It is generally an exchange system. For example, if there was an open system at 90 degrees and the air around it was at 50 degrees, heat and energy would exchange and both would reach 70 degrees. This is called an equilibrium⚖️, you'll learn more about it in unit 7.
- Closed System - A closed system is usually represented as an open system, but with a stopper. This way, air cannot transfer into or out of the system. Rather than mass and heat/energy being able to transfer, only heat is able to transfer.
- Isolated System - An isolated system is completely covered on all ends to prevent the transferring of mass, heat, and energy. An example of this is a calorimeter, which is key in this unit! Insulations allow for an isolated system as well.
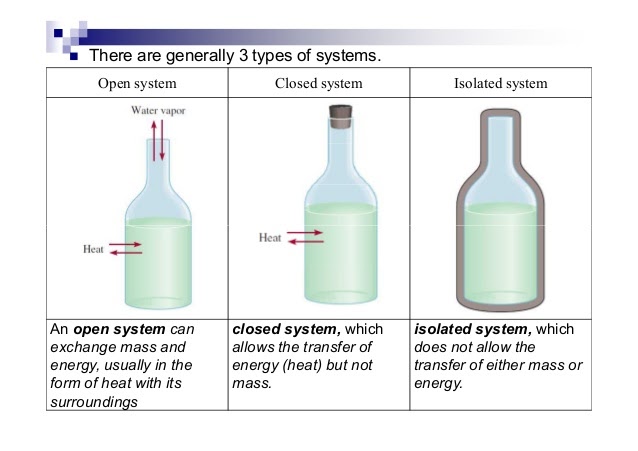
Image Courtesy of Socratic
The Surroundings
The rest of the universe outside the system, such as a beaker, constitutes the surroundings.
The system is where the reaction takes place and the surroundings are everything around the system.
State Functions
In thermodynamics, we study changes in the state of a system. State functions, such as energy, enthalpy, pressure, volume, and temperature are properties determined by the state of the system. It does not matter how the condition was achieved, so focus on the initial and final states of the system ONLY.
Heat and work are not state functions, because they vary on the path🛣️ to the destination.
Endothermic vs Exothermic
All forms of energy can be described as either exothermic or endothermic processes.
Endothermic processes🥵, as indicated by the prefix endo-, are processes where heat is supplied to the system by the surroundings. This corresponds with a +ΔH value. ΔH is just enthalpy, or heat energy.
- Remember: endo, positive ΔH, surroundings to system.
Exothermic processes🥶, as indicated by the prefix exo-, are processes that give off heat and transfer thermal energy from the system to the surroundings. This corresponds with a -ΔH value (The system is LOSING) .
- Remember: exo, negative ΔH, system to surroundings.
Think about this concept from the point of view of the system. If the system is gaining energy, there is a positive change, so it is an endothermic process. If the system is losing energy, there is a negative change, so it is an exothermic process. We'll go over ΔH more later in this unit!
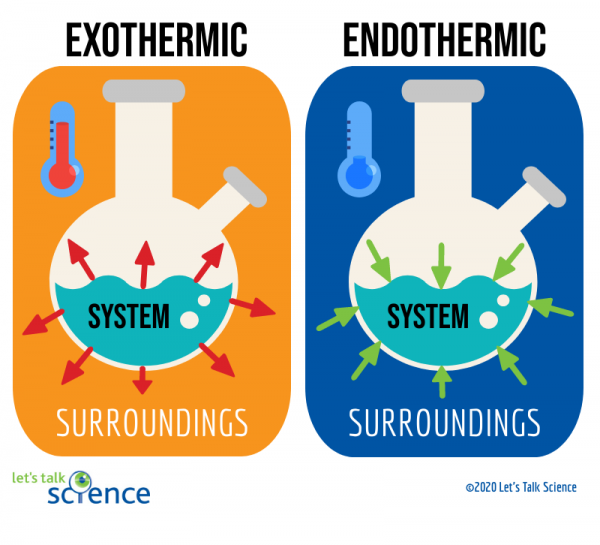
Image Courtesy of Let's Talk Science
Examples
Cold packs and hot packs, how do they work? Well, it is all about exothermic and endothermic processes.
Could you guess which process goes to which pack?
Well..endothermic processes occur in instant cold packs, and exothermic processes occur in instant hot packs.
Inside of a cold pack, there are two separate bags: one containing water and the other containing ammonium-nitrate. When you start to crack the cold pack, the barrier between the bags break and the two components react.
The reaction between water and ammonium-nitrate requires energy, so energy is pulled in from the surroundings and into the system. This is, essentially, an endothermic process.
Hot packs do the opposite!
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.