6.4 Heat Capacity and Coffee-Cup Calorimetry
5 min read•january 13, 2023
Dalia Savy
A
Anika P
Dylan Black
AP Chemistry 🧪
269 resourcesSee Units
Coffee-Cup Calorimetry
The absolute enthalpy of a system (H) cannot be measured directly. However, it is possible to measure changes in enthalpy (ΔH) by measuring temperature changes, which represent heat being lost or gained. To measure enthalpy changes, we use what is called calorimetry.
Calorimetry is a process by which a reaction takes place in a controlled vessel in which there is a liquid (typically water) and a temperature gauge like a thermometer to measure how the liquid heats up. At constant pressure, q (the amount of heat transferred to a system) is equal to delta H. We can use coffee-cup calorimetry to observe energy changes:
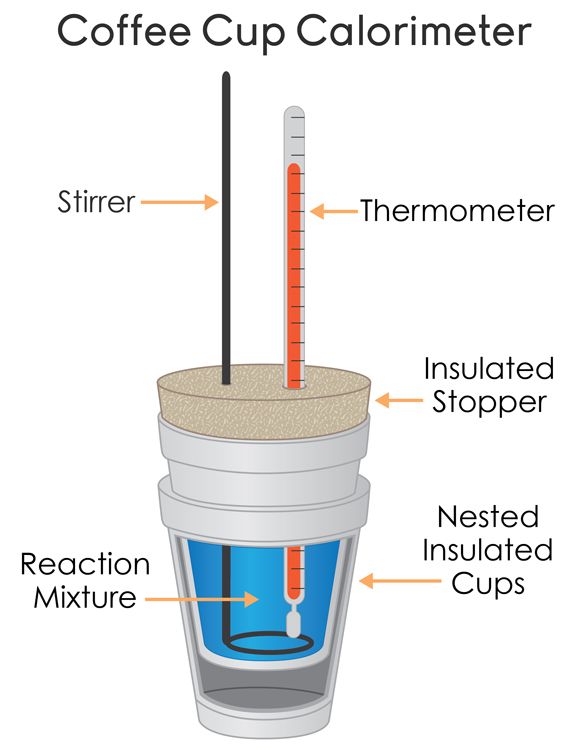
What the Heck is Going On?
We have a few parts to this coffee-cup calorimeter: we have the thermometer🌡️ and the reaction mixture, which are self explanatory, along with the stirrer, which stirs the reaction mixture. The rest of the calorimeter is meant to insulate the sample, meaning that heat cannot come in or out of the system. The more insulated, the better the measurement📏.
Measurement of Energy
In thermochemistry, heat changes are measured with calorimeters. The measurement of heat changes involves knowing specific heat, the amount of heat required to raise the temperature of a gram of a substance by 1° C. This can be used to determine heat capacity, the amount of heat required to raise the temperature of an object by 1° C.
Heat Transfer Equation: q=mCΔT
Where q is heat in joules, m is mass in grams or kilograms, C is specific heat, and ΔT is change in temperature in Kelvin (note: because they are equivalent scales, delta T for celcius is the same as delta T for Kelvin).
Specific Heat
Well...specific heat is the amount of energy required to raise the temperature of a gram of a substance by 1° C, but what the heck does this actually mean?
💧Water
Let's take a look at a real life example: boiling water. Do you ever get annoyed at the speed at which water boils? It's soooo slow, it becomes annoying to wait for when hungry.
The reason why water takes a long time to boil is because of its high specific heat. This means that water takes a long time to take in energy, or enough energy, for the water to boil. The specific heat of water is 4.184 J/g.
🏖️Sand
Now let's take a look at sand: does it heat up faster than water? When you go to the beach, you probably feel the scorching hot sand at your feet. It heats up a lot faster than water - it takes much less energy to heat sand up than it takes to heat water up. The specific heat of sand is about 0.840 J/g.
The specific heat of sand is much smaller than the specific heat of water. Therefore, we can conclude that the higher the specific heat, the more energy⚡ it takes for an object to heat up and cool down.
Usually, the specific heat of an object is given on the AP Chemistry exam. You just have to know where to plug it in (q=mCΔT is only one of the few equations in this unit😉).
Example
An insulated cup contains 255.0 grams of water and the temperature changes from 25.2° C to 90.5° C. Calculate the amount of heat released by the system. The specific heat capacity of water is 4.184 J/g°C
q = mcΔT
q = 4.184 J/g° C x 255.0 g x 65.3° C = 69,700 J = 69.7 kJ (1000J = 1kJ)
Make sure the units that you are using match! If you were to do 0.2550 kg, then the calculation would have been messed up.
Heat of reaction is an extensive property, meaning that it is dependent on mass of substance. q=mCΔT helps us determine how much heat.
Whenever you see a temperature change in a problem, think about using this formula.
Misconception
q does not equal ΔH. q is always positive, because you can't have a negative amount of energy. q is basically a magnitude of energy. ΔH could be positive or negative though; it depends on if the heat is released or absorbed.
Example #2
The following question is from the Advanced Placement YT Channel. The following laboratory procedure is being done in a calorimeter.
| Mass of Copper | 50.00g |
| Initial Temperature of Copper | 100.0°C |
| Mass of Water | 100.00g |
| Initial Temperature of Water | 20.0°C |
| Final Temperature of System (Copper and Water) | 23.6°C |
| Specific heat capacity of water | 4.18 J/g*°C |
| Specific heat capacity of copper | ? |
(a) What is |ΔT| for the copper? What is |ΔT| for the water?
Whenever you see changes in temperature, you should automatically find the change in temperature (ΔT).
ΔT is always final-initial
- Copper: |23.6°C - 100.0°C| = 76.4°C
- Water: 23.6°C - 20°C = 3.6°C
(b) A student claims that, since the magnitude of ΔT for the copper is greater than that of the water, it means that the magnitude of heat (q) lost by the copper is greater than the magnitude of heat (q) gained by the water. Do you agree with this claim?
Let's recall the first law of thermodynamics: no energy could be created or destroyed. Therefore, the heat lost by the copper must be equal to the heat gained by the water.
Sample Response: Assuming the calorimeter lost no heat to its surroundings, the heat lost by the copper must equal the heat gained by the water, despite their significant changes in temperature.
(c) Find the specific heat of copper.
Considering we only learned one formula so far, we must use q=mcΔT. In general however, if you see specific heat, the formula you have to use is most likely this one.
Since we are solving for c, we must be given q, the mass of the copper, and the change in temperature.
One problem🤔: we don't have q! Let's go back to part b...the magnitude of heat lost by the copper is equal to the magnitude of heat gained by the water.
This enables us to use q=mcΔT twice, by setting the water calculation equal to the copper calculation (q for copper is equal to q for water so what q equals for copper must be equivalent to what q equals for water).
The set up looks like this:
mcΔT (copper) = mcΔT (water)
(50.00g)(c)(76.4°C) = (100.00g)(4.18 J/g*°C)(3.6°C)
c = 0.39 J/g*°C
Another Formula
So we know that we could use q=mcΔT, but when we have a heat loss and heat gain question, we could also use:
heat loss = heat gain or mcΔT (1st substance) = mcΔT (2nd substance)
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.