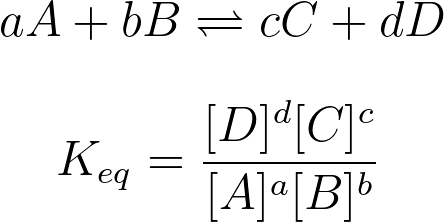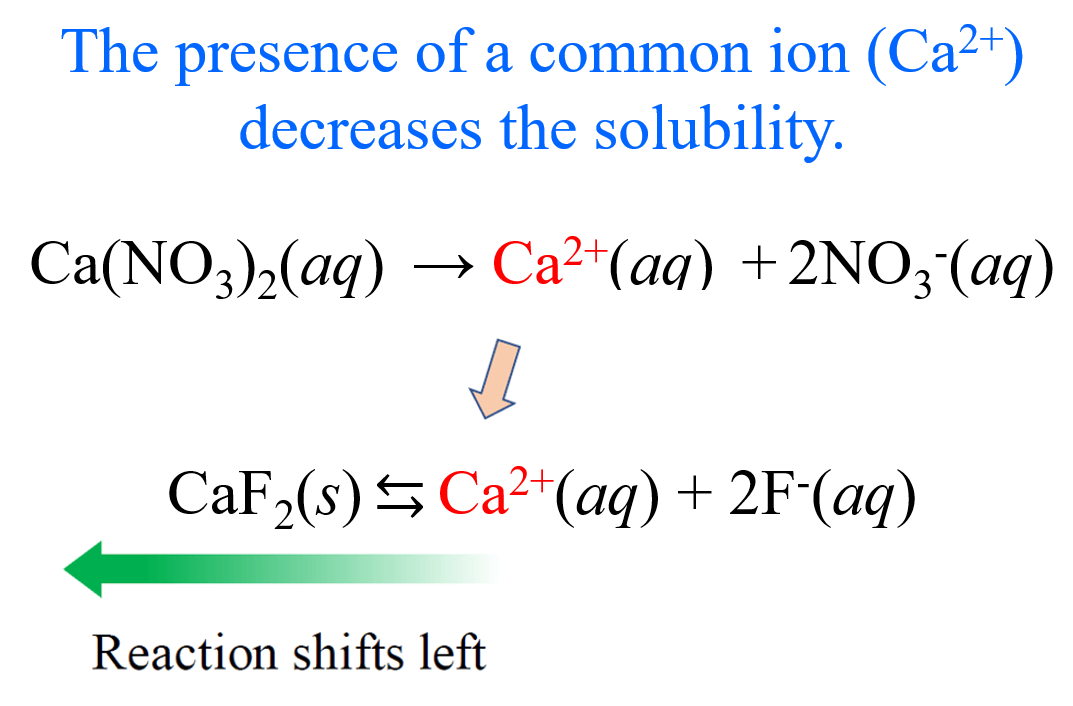According to the College Board, "Chemical equilibrium is a dynamic state in which opposing processes occur at the same rate. In this unit, students learn that any bond or intermolecular attraction that can be formed can be broken. These two processes are in a dynamic competition, sensitive to initial conditions and external perturbations. A change in conditions, such as addition of a chemical species, change in temperature, or change in volume, can cause the rate of the forward and reverse reactions to fall out of balance.
Le Châtelier’s principle provides a means to reason qualitatively about the direction of the shift in an equilibrium system resulting from various possible stresses. The expression for the equilibrium constant, K, is a mathematical expression that describes the equilibrium state associated with a chemical change. An analogous expression for the reaction quotient, Q, describes a chemical reaction at any point, enabling a comparison to the equilibrium state. Subsequent units will explore equilibrium constants that arise from acid-base chemistry."
Why is a waterfall considered a spontaneous reaction?
How can reactions occur in more than one direction?
How is caffeine removed from coffee? ☕
Why is food stored in a refrigerator? ❄️
In Unit 5, we brought up the idea of kinetics, the study of the rate of a reaction. We determined that there are many factors such as concentration and temperature that can increase or decrease the rate of a reaction. In unit 7, which focuses on a new topic called equilibrium, we’ll look at the rate of a reaction going forwards➡️ and backward⬅️. In equilibrium, we discuss reactions that are reversible meaning that not only are products created from reactants but reactants can also be created from products! In fact, nearly every reaction is reversible. The study of equilibrium describes how product-favored or reactant-favored these reversible reactions are.
Our essential question for this unit is “how can we determine, both qualitatively and quantitatively, how far forward a reaction goes and how we can impact its equilibrium?” This question will be answered by looking into theories such as Le Chatelier’s Principle and mathematically by calculating equilibrium constants and equilibrium concentrations. By looking at both the qualitative and quantitative aspects of equilibrium, we’ll be able to get a better view of what actually happens during a reaction and why some reactions may seem not to happen but in fact, only happen a little bit.
Let’s start this unit out with a broaaaad overview of what equilibrium is all about. In chemistry, we define equilibrium as the point at which the rate of the forward reaction is equal to the rate of the reverse reaction. We learned about rates in unit five as how quickly a reaction proceeds. We can also think of this rate as a change in concentration of the products (or reactants) over a change in time: R = Δ[P]/ΔT = -Δ[R]/ΔT. Because of this, our study of equilibrium will primarily deal with changes in concentration up to equilibrium.
With reverse reactions, we see the same just in the opposite direction! Reversible reactions can be described as two reactions in one: one going forward and the other going backward. We notate this as reactants ⇌ products, the double arrow indicating to us that both reactions are occurring simultaneously. Therefore, we have two reactions actually occurring: reactants → products and products → reactants.
At equilibrium, as we mentioned, the rates of both of these reactions are the same meaning that the change in concentration of the products over time equals the change in concentration of the reactants over time. This tells us two things: first that there are no more changes in concentrations of either (because we are losing and gaining at the same rate) and that equilibrium is not when a reaction “stops!" Equilibrium is a dynamic process by which rates of reactions settle, not stop!
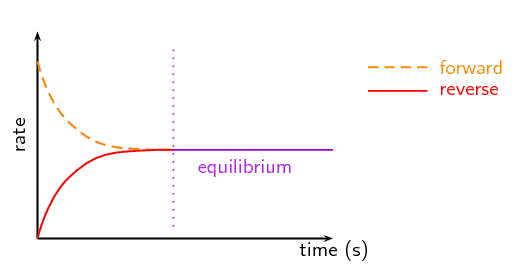
Image From Siyavula
Take a look at the above image. We see two rates: the rate of the forward reaction in dashed lines and the reverse reaction in the solid line. Over time, we can see that these rates become equal. Because they are equal we say that our reaction is in equilibrium. This idea of equal forward and backward rates drives all of our discussions in this unit!
Moving into the math of this unit, you'll learn how to calculate the reaction quotient, Q, and the equilibrium constant, K. When doing calculations with equilibrium, we use the equilibrium constant, K for a reaction. K describes the ratio between the rate of the forward reaction and the rate of the reverse reaction, as well as the ratio between the equilibrium concentrations of products and reactants.
The mathematical formula for K is very similar to that of Q, the reaction quotient. However, when calculating with Q, the concentrations we use are concentrations at a certain time t 🕔 whereas when calculating K we must use equilibrium concentrations. We'll dig deeper into what this means, but we can use these two quantities to tell where a reaction is standing with regard to equilibrium.
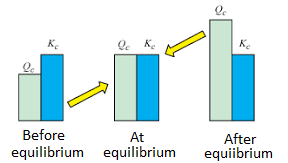
Image Courtesy of Byjus
When calculating equilibrium constants, you are typically given equilibrium concentrations or are given an easy way to calculate them without using K (however, the former is much more common). At that point, you simply plug into one of the formulas we’ve learned and get your answer!
There are two key points in this study guide, along with a ton of practice:
K, the equilibrium constant, is a unitless quantity.
Solid precipitates and liquids are not part of the equilibrium expression, only aqueous solutions and gases are.
Image Courtesy of Study.com
Since Kc is simply a ratio of the amount of product at equilibrium to the amount of reactant at equilibrium, we can realize a few things:
If K > 1, the reaction is product-favored, meaning that we produce more products at the end than we had at the beginning.
If K = 1, the reaction is neither product-favored nor reactant-favored, meaning the system is at equilibrium. The concentrations of the reactants and products remain constant when K = 1.
If K < 1, the reaction is reactant-favored, meaning that the reverse reaction is preferred to the forward reaction.
We'll dig into what this means deeper in this study guide, but for now, here is a quick overview!
In Unit 6, we discussed Hess’s Law and how it can be used to find the value of ΔH for reactions by adding together, flipping, and multiplying reactions. In this section, we’ll apply similar rules to equilibrium!
If the reaction is reversed, you must inverse the value of K (take its reciprocal).
If the reaction is multiplied by a constant (n), you must raise the equilibrium constant to the power, n.
If you add several reactions together, you multiply their equilibrium constants.
In general, if you did well with Hess's Law problems, then they should be a pretty good lesson!
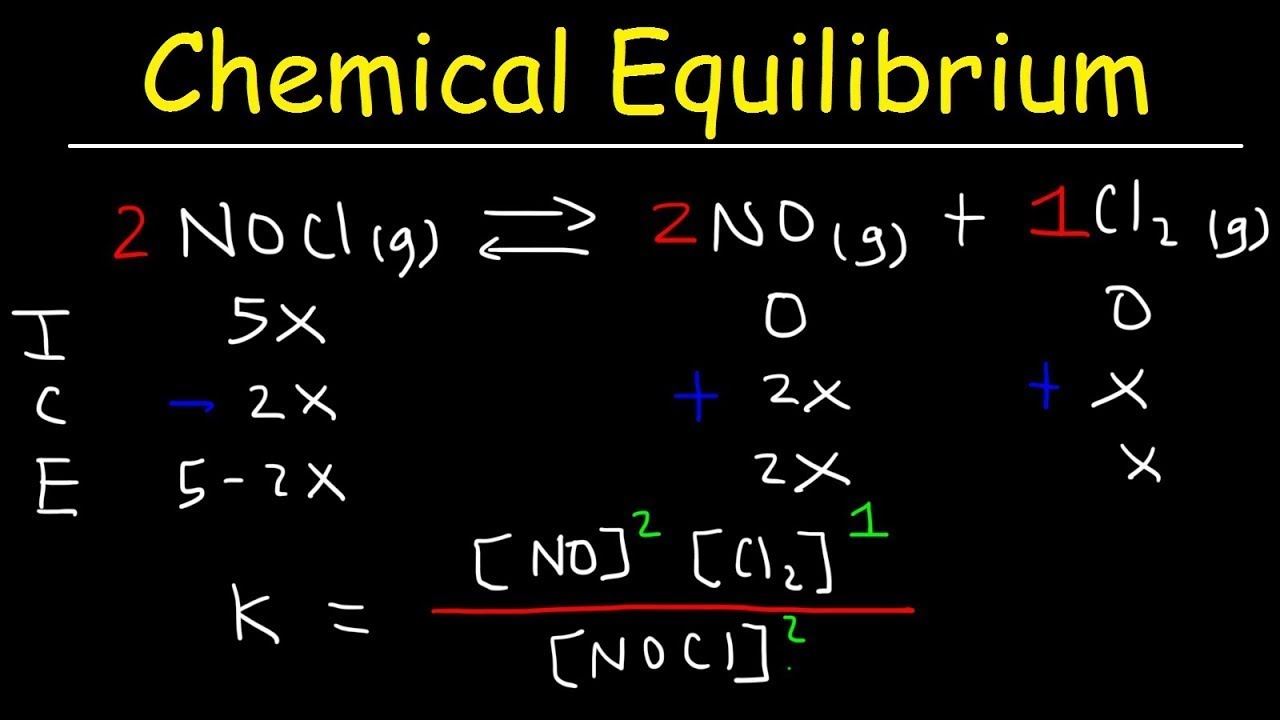
Once we have a strong foundation on equilibrium as a whole, we can start discussing how to find concentrations at equilibrium. This is where the unit gets fun! In this study guide, you’ll start using ICE Boxes, a tool that you’ll carry with you for the rest of your chemistry career (which of course will last a lifetime because you’re going to study chemistry forever, right? right?). These will be used to find what the concentrations of both reactants and products are at equilibrium by using equilibrium constants.
Image Courtesy of the Organic Chemistry Tutor
The majority of unit seven focuses on mathematical representations of equilibrium. We’ll see this in the form of calculating equilibrium constants and using ICE Boxes to calculate equilibrium concentrations. However, this unit incorporates qualitative analyses of equilibrium as well. In fact, understanding equilibrium conceptually is a super powerful tool because not only does it mean the formulas and math will make more sense, but it also means that you’ll be able to properly explain your answers which is invaluable on FRQs. The College Board also loves to test conceptual knowledge on equilibrium on MCQs where they can give separate answers for a question, and on FRQs, where they can ask you to draw particle diagrams!
You’ll also learn about Le Châtelier’s Principle, which helps describe how reactions return to equilibrium from an external change like changes in concentrations or temperature. Le Châtelier’s principle acts similar to a seesaw!
Image Courtesy of SlideShare
Le Châtelier's Principle states that if a chemical system at equilibrium is subjected to a stress, it will shift in a direction that tends to alleviate that stress. You'll not only learn how this principle operates, but why it does as well, which brings us to the next key topic!
In this study guide, we'll discuss the relationships between Q, K, and the direction in which a reaction will shift to reach equilibrium. This way of thinking about Q vs. K will help guide our mathematical justification of Le Châtelier’s Principle for concentration and pressure, but we’ll also see how temperature stands out as an exception to using Q to justify. Hint hint: K is a temperature-dependent value.
Finally, in the last few key topics, we’ll apply our newfound expertise on equilibrium to a topic that we learned all the way back in
unit four: solubility! Before, we spend a lot of time deciding whether a solute can dissolve in a solvent, but after discussing equilibrium, we can apply these ideas to the dissolution of various compounds that we previously classified as insoluble.
We typically describe insolubility as the quality of a substance to not break down in a solvent at all. However, we’ll find that insoluble compounds do dissolve, it’s just that their equilibrium constants are suuuper small, so very little ends up dissolving.
The common ion effect describes how a common ion can suppress the solubility of a substance. This phenomenon occurs when a substance with a common ion (an ion that is present in two or more different compounds) is added to a solution containing a salt of that ion. This can affect the equilibrium of the solution and cause the concentration of the ion to change!
We'll combine our knowledge of ICE tables with the common ion effect to see how exactly common ions change the solubility of a substance.
Image Courtesy of Chemistry Steps
Ideas of pH and solubility connect directly to the common ion effect and Le Chatelier’s Principle. Remember that pH describes the concentration of hydrogen ions (H+) and, conversely, hydroxide ions (OH-) in a solution. By observing connections between solubility equilibria, Le Chatelier’s Principle, and ion concentrations, we will be able to achieve some insights as to how pH can impact solubility equilibria!
The final section of unit seven brings together two topics: solubility and thermodynamics, which in AP Chemistry can be defined as the study of energy transfers during chemical reactions. Note that we used the word energy and not a word like “temperature” or “heat.” This is because heat is only one component of thermodynamics.
We're basically going to discuss the solubility of a salt in relation to the changes in enthalpy and entropy that occur in the process of dissolution.
Equilibrium - a state where the rate of the forward and reverse reactions of a chemical system are equal and the concentrations of the reactants and products remain constant.
Reversible reaction - a chemical reaction that can proceed in both the forward and reverse direction and reaches an equilibrium state.
Equilibrium constant - a value that describes the relative concentrations of reactants and products at equilibrium in a chemical reaction.
Closed system - a system that does not exchange matter or energy with its surroundings.
Equilibrium concentrations - the concentrations of reactants and products at equilibrium in a chemical reaction.
Dynamic equilibrium - a state of balance in which the concentrations of reactants and products in a chemical reaction remain constant over time, due to the forward and reverse reactions proceeding at equal rates.
Reaction quotient - a value that describes the relative concentrations of reactants and products in a chemical reaction, before the reaction reaches equilibrium.
Particulate models - representations of chemical reactions using symbols and formulas to represent the individual particles involved in the reaction.
Le Châtelier’s Principle - a principle that states that if a chemical system at equilibrium is subjected to a stress, it will shift in a direction that tends to alleviate that stress.
Insoluble - a substance that does not dissolve in a solvent...but we'll be adjusting this definition!
Solubility product - a value that describes the equilibrium constant for the dissolution of a slightly soluble salt in water.
Molar solubility - the number of moles of a solute that will dissolve in one liter of solvent to reach saturation.
Common-ion effect - the reduction of solubility of a salt caused by the presence of an ion that is already in the solution.
pH - a measure of the acidity or basicity of a solution, on a scale from 0 to 14.
Free energy change - a measure of the change in the amount of energy available to do work in a chemical reaction.