7.4 Calculating the Equilibrium Constant
3 min read•june 1, 2021
Dylan Black
AP Chemistry 🧪
269 resourcesSee Units
7.4: Calculating the Equilibrium Constant
When calculating equilibrium constants, you are typically (read always) given equilibrium concentrations or are given an easy way to calculate them without using K (however the former is much more common). At that point, you simply plug into one of the formulas we’ve learned and get your answer! Remember, K is a unitless quantity. It is important to note however that solid precipitates and liquids are not part of the equilibrium expression.
Formulas For Equilibrium Constants
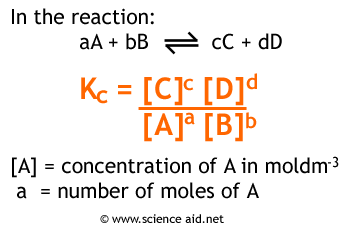

Example with Kc
Calculate 🖩 the value of the equilibrium constant, Kc, for the system shown, if 0.1908 moles of CO2, 0.0908 moles of H2, 0.0092 moles of CO, and 0.0092 moles of H2O vapor were present in a 2.00 L reaction vessel were present at equilibrium.
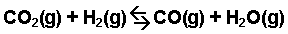
First, begin by writing out the Kc expression for this reaction:
Kc = [CO][H2O]/[CO2][H2]
Then find our equilibrium concentrations by dividing by 2.00L:
CO: 0.0092/2 = 0.0046
H2O: 0.0092/2 = 0.0046
CO2: 0.1908/2 = 0.0954
H2: 0.0908/2 = 0.0454
Finally, we can plug into the formula above to find:
Kc = [0.0046][0.0046]/[0.0954][0.0454] = 4.9 * 10^(-3).
Example with Kp
Calculate the Kp for the reaction:
2N2O5 ⇌ O2 + 4NO2
P(N2O5) = 2.00
P(O2) = 0.296
P(NO2) = 1.70
First we can write out our Kp expression
Kp = P(O2)P(NO2)^4 / P(N2O5)^2 = (0.296)(1.70)^4 / 2.00^2 = 0.618
Justifying the Formula For The Equilibrium Constant
Let’s think about why the formula we’ve been using actually works. In a general reversible reaction A + B ⇌ C + D, the equilibrium constant K is equal to the ratio of the equilibrium concentrations of the products raised to their stoichiometric coefficients to the equilibrium concentrations of the reactants raised to their stoichiometric coefficients. We see this mathematically as K = [C][D] / [A][B]. Let’s think about what this formula actually tells us. Recall that these concentrations are equilibrium concentrations meaning the numbers we plug into this formula are after the reaction reaches equilibrium. By finding a ratio we’re essentially asking the question “how does the number of products at equilibrium compare to the number of reactants at equilibrium?”. This helps us explain why a K value above 1 indicates a product favored reaction and vice versa. The formula tells us that when K is over 1 [C][D] > [A][B] meaning that we now have more product than reactant. Similarly, when K is less than 1 [C][D] < [A][B] and thus we still have a large amount of reactant. Note that K can never be negative but can be extremely small. This way of thinking can help you understand why the equilibrium constant formula is the way it is!
Tips When Calculating Equilibrium Constants
While calculating equilibrium constants is usually a plug and play game, there are a few things you want to be careful of before blindly plugging into the formula. The most important aspect of the formula is that concentrations and pressures are so AT EQUILIBRIUM! Plugging in pressures at any other point besides at equilibrium will calculate Q, the reaction quotient, which for all but ONE point is not the equilibrium constant. Thus you have to be super careful that you are actually plugging in values at equilibrium.
You also want to make sure the numbers you’re plugging in are actually concentrations/pressures. For example, look back to the example for calculating Kc. We glossed over this step because it’s assumed prerequisite knowledge for this unit but you want to make sure that you are converting to the proper units. Using the example we went through as a sample, we see that we had to divide by 2.00L to find mol/L because we were originally given moles. A problem could in theory take this a step further and give you grams and expect you to convert grams to moles and then moles to moles per liter. Always be prepared to make unit conversions when you have to. This could also take the form in calculating partial pressures. For example if you were given a total pressure and then moles of each gas you would have to use PA=XA*Ptotal to find each partial pressure and then plug into the Kp expression. These instances may be rare but could theoretically pop up because they are part of chemistry.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.