7.5 Magnitude of the Equilibrium Constant
4 min read•june 1, 2021
Dylan Black
AP Chemistry 🧪
269 resourcesSee Units
7.5: Magnitude of the Equilibrium Constant
So far in unit 7 we’ve discussed what equilibrium is and how equilibrium constants can help us describe how far forward a reaction goes. However let’s dive a little deeper into what the equilibrium constant actually means.
What Exactly Does The Equilibrium Constant Tell Us?
To understand the equilibrium constant in more depth, let’s take a peek back at the formula:
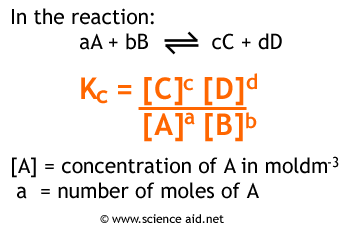
(PS: Everything we discuss in this guide about Kc also pertains to Kp!)
This formula looks unwieldy and a bit clunky at first, but let’s break down what it actually means. In the numerator, we have the concentrations of the products raised to their stoichiometric coefficients. This value can be understood as a representative of the amount of product we have at equilibrium. Similarly, the denominator serves the same purpose just for the reactants. Therefore, Kc is simply a ratio of the amount of product at equilibrium compared to the amount of reactant at equilibrium. This means a few things:
If K > 1, the reaction is product favored, meaning that we produce more products at the end than we had at the beginning (Assuming we have starting conditions before equilibrium). We also know that the greater K is, the farther forward the reaction goes because it would imply a higher ratio of product to the reactant. For example, a reaction with a K of 2 is still product favored, but a reaction with a K of 10^12 is much more so.
If K = 1, the reaction is neither product favored or reactant favored. When K = 1, that would imply that the product of the product concentrations and the product of the reactant concentrations are equal, meaning that the reaction equally produced product and reactant at equilibrium.
If K < 1, the reaction is reactant favored, meaning that the reverse reaction is preferred to the forward reaction. The reaction will still produce the product, but the vast majority of it will re-react to form the products meaning there will be incredibly low concentrations of the product by equilibrium. By the end, we would have most of the product we started with with a tiny tiny amount of product created. Like when K > 1, the lower K is, the less a reaction goes forward. Note that K can never be negative.
Comparing Reactions Based On Equilibrium Constants
Using this new knowledge, we can also draw comparisons between equations by comparing their equilibrium constants to see which reaction goes further forward. For example, consider these two reactions involving the dissociation of two acids:
CH3COOH ⇌ CH3COO- + H+ (K = 1.8 * 10^(-5))
HCl ⇌ Cl- + H+ (K = 1.3 * 10^6)
Which of the following acids will release more H+ into a solution with the same starting concentration?
Without doing any calculations (which we’ll learn how to do in 7.7), we can see that the K value for the dissociation of HCl is well over 1 meaning we will have more product than reactant at the end whereas with CH3COOH most of our acid will remain undissociated because its equilibrium constant is way below 1. Therefore, our answer would be HCl.
Chloride and H3O+, the products of the dissociation of HCl. Image from Wikipedia
Practice Problem
- Identify each of the following reactions as either product favored or reactant favored:
- CH3COOH ⇌ CH3COO- + H+ (K = 1.8 * 10^(-5))
- 2O3 ⇌ 3O2 (K = 2.5 * 10^12)
Let’s go through each of these equations and see if we can figure out how far forward the reactions go by observing their equilibrium constants. Equation (a) is the same equation we looked at earlier in this guide. We can see that the K value for the dissolution of acetic acid is less than 1 meaning that this reaction will not go very far forward. This tells us that the reaction is reactant favored. From this conclusion we can find that in a solution of CH3COOH we will have mostly acetic acid and not much acetate ions and H+ ions. However, let’s take a look at reaction (b). This reaction is the decomposition of O3 also known as ozone into molecular oxygen.
Fun Fact! This reaction takes place in the atmosphere and is catalyzed by chlorine! This is why if you’ve ever heard of CFCs (chlorofluorocarbons) they’re seen as bad for the environment. These compounds are found in hairspray and other aerosol products. The chlorine serves as a catalyst and speeds up the decomposition of ozone which is why during the 1980s we saw the growth of a hole in the ozone layer.
We see that this reaction has a K value wayyyy above one. This means that the reaction goes nearly all the way forward telling us that at the end we will have mostly product and not very much reactant. This means the decomposition of ozone is product favored.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.