7.3 Reaction Quotient and Equilibrium Constant
4 min read•january 13, 2023
Dylan Black
AP Chemistry 🧪
269 resourcesSee Units
7.3: Reaction Quotient and Equilibrium Constant
So far we’ve talked exclusively about reactions at equilibrium. However, reactions can be anywhere! They can be pre-equilibrium, post-equilibrium, or at equilibrium. We can describe where a reaction is in the process of reaching equilibrium by calculating Q, the reaction quotient.
What is Q, the Reaction Quotient?
To start explaining what Q means, let’s take a look at the formula:
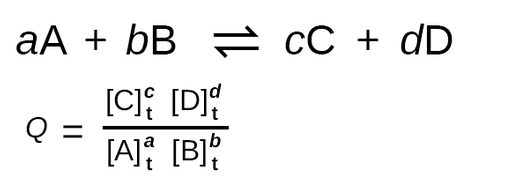
Wait wait wait 👀… back up… isn’t that the formula for the equilibrium constant K? If you thought that upon reading the formula you’d be mostly correct! However, there’s a super important distinction to be made. When calculating with Q, the concentrations we use are concentrations at a certain time t 🕔 whereas when calculating K we must use equilibrium concentrations. Therefore, Q describes the ratio of products to reactants at any point in the reaction. By comparison, K describes the ratio of products to reactants at equilibrium specifically. By using Q we can predict whether a reaction will go further forward (produce more products), reverse (produce more reactants), or stay the same (we’re at equilibrium!).
Q will serve to be an incredibly important concept both mathematically and qualitatively throughout the rest of unit 7. Near the latter half of the unit you will start learning about how equilibria will react to external changes such as changes in concentration, temperature, pressures, etc. The primary justification for most of these changes (temperature being the notable exception) will be changes in Q! Q helps guide us in finding out where a reaction “is” in the process of reaching equilibrium whether we haven’t quite gotten there yet, we’re there, or if we overshot and have to go backwards to return.
Understanding Equilibrium Concentrations vs. non-Equilibrium Concentrations
We’ve introduced the idea of concentrations not at equilibrium. These are concentrations that are either before or after equilibrium. At these concentrations, a reaction will readjust itself to equilibrium, either creating new products or creating new reactants to return to equilibrium (this is why equilibrium is called equilibrium!). The shift that Q takes to return to K is similar to a seesaw. If there is too much weight on one side the seesaw can add weight to the other side to balance out! This analogy can help you visualize how Q shifts back towards equilibrium by either creating products or creating reactants to shift the reaction right or left.
Comparing Q and K
The most important aspect of using Q and K to solve problems is stating what direction a reaction will move. There are three scenarios we have:
When Q > K, there are more products than reactants (concentration wise) compared to K. To decrease the amount of products, the reaction will shift to the left and produce more reactants.
When Q = K, we’re at equilibrium! This means that there will be no change in concentrations.
When Q < K, there are more reactants than products (concentration wise) compared to K. As a result, some of the reactants will become products, causing the reaction to shift to the right 👉.

Mathematical Justification
We can also understand the relationship between Q and K mathematically. We know that for A + B ⇌ C + D, Q = [C]t[D]t/[A]t[B]t. When Q > K we know that [C]t[D]t will be too high compared to the value at K meaning it needs to decrease and [A]t[B]t must increase in order to return to equilibrium. Therefore the reaction will use the excess C and D to create A and B until the system is at equilibrium. This thinking can also be applied to the opposite scenario where Q < K. If Q < K that means [C]t[D]t is smaller than it should be and [A]t[B]t is larger than it should be. Thus, in order to return to equilibrium the excess reactant will react to form the product and stabilize Q to return to K. This mathematical analysis of Q matches our qualitative ‘seesaw’ analogy from before.
Practice Problem
Consider the following reaction:
2NOBr ⇌ 2NO + Br2
If Kc= 0.0142 and the initial concentrations are 1.0 M NOBr, 0.2M NO, and 0.8M Br2, which way will the reaction progress to reach equilibrium?
Let’s use Q to calculate whether or not we will move left or right:
Q = [NO]^2[Br2]/[NOBr]^2 = (0.2)^2(0.8)/(1)^2 = 0.032.
0.032 > 0.0142 ⇒ Q > K ⇒ the reaction will proceed to the left because we have passed K.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.