7.9 Introduction to Le Chatelier's Principle
4 min read•january 13, 2023
Dylan Black
AP Chemistry 🧪
269 resourcesSee Units
7.9: Introduction to Le Chatelier's Principle
Equilibrium thus far has considered only reactions going straight to equilibrium and then staying there. However, what if we wanted to say, shift an equilibrium towards the products or towards the reactants? How does equilibrium shift based on certain stressors that are added to a system? This section will address that very question by introducing a new rule: Le Chatelier’s Principle.
What Is Le Chatelier’s Principle?
Le Chatelier’s Principle states one key rule about how a system in equilibrium will react to an external pressure: “if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to counteract the change to reestablish an equilibrium” (LibreTexts). Let’s break down each part of this.
We begin by establishing that we are in a dynamic equilibrium. This just means that we have some reaction that is at equilibrium. We call it dynamic equilibrium because equilibrium does not mean the reaction has stopped but rather that the concentrations of reactants and products have not changed.
Next, our dynamic equilibrium is disturbed by “changing the conditions”. We’ll cover some specific disturbances in the next section, but generally this phrase refers to any external change that comes to the system from outside. This could mean adding more of something, increasing temperature, changing pressures, etc.
Finally, and this is the part where Le Chatelier’s Principle answers our question, the equilibrium will shift to counteract the change and re-establish equilibrium. Think of Le Chatelier’s principle like a seesaw that always wants to remain in balance. If you add a 50-pound weight to one side, Le Chatelier’s principle will respond by counteracting that change and shifting some of that 50lbs to the other side.
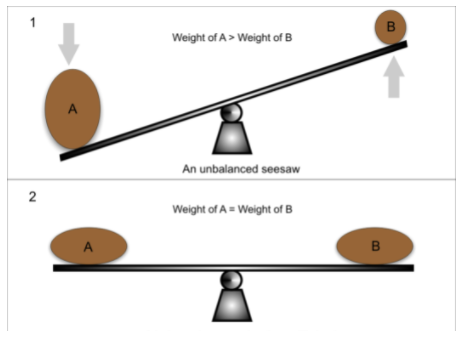
Image From Greater Minds Tutors
Factors That Influence Equilibrium
Concentration
Changes in concentration are the clearest way in which equilibrium can shift. When a system is in equilibrium, we can either add some product or add some reactant to change the equilibrium concentrations of the system. Note that our K value is not changing, just the concentrations at equilibrium because we have induced a new stress to the system. Based on Le Chatelier's Principle, we can figure out which direction.
If we add more reactant to the system, the system will respond by counteracting that change by creating more product to return to equilibrium. Similarly, if we add product to a system the system will respond
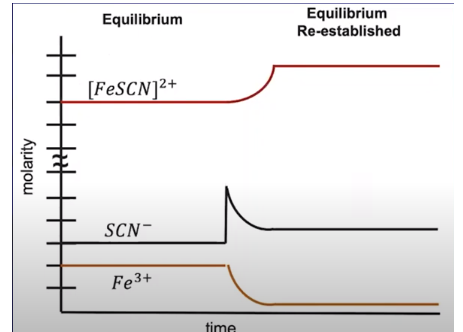
For example, consider the reaction Fe3+ + SCN- ⇌ FeSCN2+. Imagine if at equilibrium we added a compound containing the SCN- ion to the system increasing its concentration. As we can see from the below graph, we see a decrease in [Fe3+] and [SCN-] as responses to the stress whereas the system responds by creating more [FeSCN]2+.
Another important part of how concentration impacts equilibrium is adding a compound that will react with a reactant or product to take it out of the system. For example, adding a compound containing hydroxide will cause the hydroxide to react to form Fe(OH)3, taking the Fe3+ ion out of the system. Based on Le Chatelier’s principle, the system will respond by increasing the amount of Fe3+ and SCN- present in the system and decreasing the concentration of [FeSCN]2+.
Temperature
Temperature changes, like changes in concentration, have an impact on equilibrium. In this case, the change in equilibrium depends on whether or not our reaction is exothermic (heat releasing) or endothermic (heat absorbing). Remember from unit 6 that we can identify whether a reaction is exothermic or endothermic by observing its change in standard enthalpy, or ΔH°. If ΔH° < 0 our reaction is exothermic and if ΔH° > 0 our reaction is endothermic. When referring to Le Chatelier’s Principle we can think of heat as either a reactant or product and then refer to concentration for similar conclusions for temperature.
For example, consider the reaction N2 + 3H2 ⇌ 2NH3. This reaction has a ΔH° of -92 kJ/mol. Therefore, our reaction is exothermic implying that heat can be thought of as a product. This means that if the temperature increases our equilibrium will shift to produce more reactants. The opposite would occur if the reaction was endothermic and the temperature was a reactant.
Pressure
When dealing with a system involving gases, changes in pressure (and therefore the volume, remember Boyle’s Law!) will change concentrations of species in the system.
In general, if the pressure on a system increases or the volume decreases, the equilibrium will shift towards the side with fewer moles of gas and vice versa. If there is no difference in moles of gas, there will be no change either way.
When we talk about moles of gas, we’re talking about the coefficients in the reaction.
For example, consider the reaction A + B ⇌ 2C + 3D where A B C and D are gasses. On the left, there are 2 moles of gas because 1 + 1 = 2. On the right, there are 5 moles because 2 + 3 = 5. Therefore if the pressure were to increase on the system, equilibrium would shift right and products would be converted into more reactants.
However, there is one caveat to this rule. If an inert gas, that is a gas that does not react, is added to a vessel, there is no impact on equilibrium. For example, if Helium gas was pumped in, there would be no impact as far as equilibrium is concerned.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.