Dylan Black
AP Chemistry 🧪
269 resourcesSee Units
7.1: Introduction to Equilibrium
Welcome to the introduction to equilibrium! Equilibrium is an incredibly important topic both for the AP exam and for chemistry in general. Equilibrium takes what you know about reactions from units 4 and 5 and expands it into the realm of reversible reactions. That is reactions that can go both forward (Reactants turning into products) and backward (Products turning into reactants). This unit will focus on describing how reactions can do this and to what extent they do.
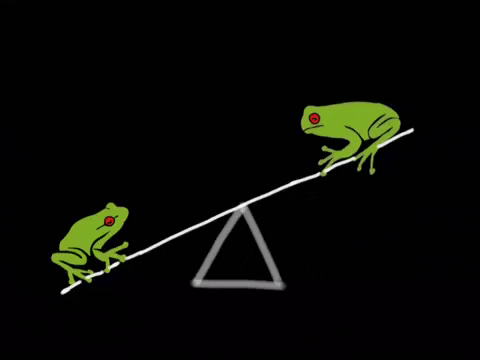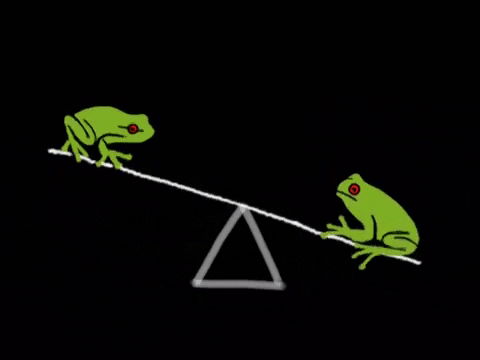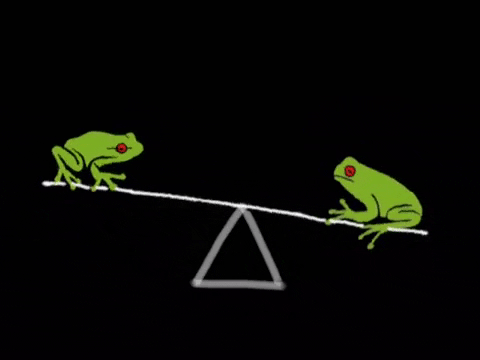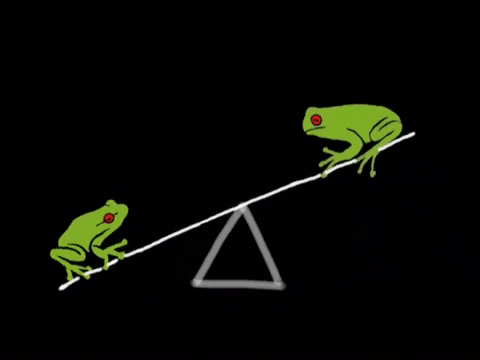
What Is Equilibrium?
Let’s begin by asking the question, “what even is equilibrium?”. In most textbooks, equilibrium is defined as the point in a reaction where the rate of the forward reaction is equal to the rate of the reverse reaction. Let’s break this down a bit. Suppose we have a reaction in which A, a reactant, is turned into B, a product. As concentrations of A decrease, the rate of the reaction A → B will decrease. However, there will be a secondary reaction, that being the reaction B → A as concentrations of B build up. In many instances, this second reaction is incredibly slow and does not happen much. However, in many other instances, this second reaction is actually the driving reaction. We’ll talk a bit more about how to measure how “far forward” a reaction goes by using equilibrium constants.
We can represent the two reactions by using a double arrow on the reaction A → B to describe that this reaction is reversible. A ⇌ B. This notation tells us that this reaction will settle at an equilibrium. Now that we’ve covered that, we’re ready to dive more into how the rates of these two reactions relate.
Like we mentioned before, equilibrium is the point at which the forward reaction and reverse reactions continue at the same rate. At this point, the production of products equals the production of reactants and so concentrations of the two remain the same. The below graphic shows us what happens at equilibrium:
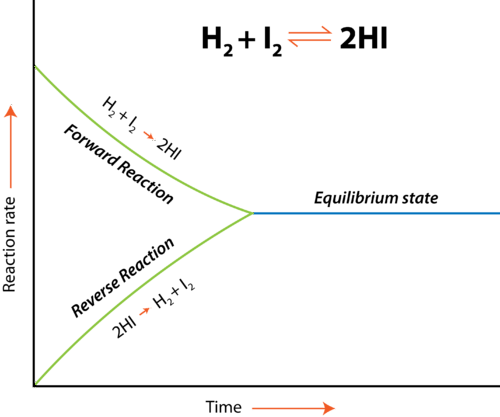
In this example, we have the equilibrium reaction H2+I2 ⇌ 2HI. We have two reactions each occurring at different rates, one being the forward reaction and the other being the reverse reaction. However, note the point at which the two become the same. That area is called the equilibrium state. At this point, the rate at which products are created equals the rate at which reactants are created and therefore the concentrations present in your reaction are equal. It’s important to note that equilibrium doesn’t mean the concentrations themselves are the same, but rather just the rates of each reaction.
Measuring Equilibrium: Kp and Kc
Now that we understand what equilibrium means in terms of reaction rate, let’s discuss how we actually measure how “far forward” a reaction goes. When doing calculations with equilibrium, we use the equilibrium constant, K for a reaction. K describes the ratio between the rate of the forward reaction vs. the rate of the reverse reaction as well as the ratio between the equilibrium concentrations of products and reactants. If we have the reversible reaction A ⇌ B, we can describe how far forward this reaction goes before equilibrium by writing out the rate laws for the forward and reverse reaction:
A → B ⇒ R = k1[A]
B → A ⇒ R = k2[B]
At equilibrium, k1[A] = k2[B]. We can describe the ratio k1/k2 as a representation of how far forward the product-creating reaction goes vs. how far forward the reactant-creating reaction goes. We can also represent this value as [B]/[A] where [B] and [A] are the concentrations of B and A at equilibrium. This value is known as Kc or sometimes simply K. The formula for K is as follows:
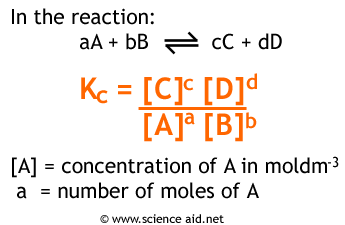
K is a unitless value that also changes based on temperature (this is because k1 and k2 will change based on temperature!). However, the starting concentrations do NOT change K. No matter where you start a reaction (assuming constant temperature) you will find that K remains constant. This will also be true for KP which we’ll discuss next.
There is another way of measuring equilibrium, specifically when discussing gasses. Like we use concentrations, we can also use partial pressures to describe how many products were formed and how many reactants remain at equilibrium. This value is known as Kp. Kp is the same calculation as Kc just with partial pressures instead of concentrations.
For both of these values, examples of calculating them will be covered in unit 7.4, but make sure you’re comfortable understanding what they mean in a non-mathematical way as well like we’ve described here.
There is also a relationship between Kp and Kc that is described by the formula Kp= Kc(RT)^(Δn) where Δn denotes the difference in stoichiometric coefficients of the products and the stoichiometric coefficients of the reactants. This won’t be too common on the exam (in fact it probably won’t show up) but it’s nice to know!
Common Misconception: Equilibrium = Stopped Reaction
Let’s close this guide out with a common misconception that students have about equilibrium that hinders their understanding of equilibrium as a balancing of rates. Many students believe that at equilibrium the reaction stops completely. This could not be further from the truth! In fact, reversible reactions never “end”. Equilibrium is a dynamic process in which reactants are still turned to products and vice versa, not the point at which a reaction stops.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.